2018 Joint Commission ORYX Requirements
By Erin Heilman October 13, 2017 Regulatory Updates: Hospital

In 2018, the Joint Commission has decided not to change any of their requirements for the ORYX® initiative for quality improvement program. In this blog, we will review The 2018 Joint Commission ORYX requirements for accredited hospitals. Keep in mind that these requirements are the same for 2017 and 2018 reporting years.
The ORYX® initiative was started to incorporate performance data into the Hospital Accreditation Program. Both chart-abstracted and electronic measures are now required for successful completion of the program.
In 2015, eCQMs were introduced into the program for the first time. However, 2017 marked the first year that eCQMs are required for successful completion of the program. Up until this year, only chart-abstracted measure sets were required. Now the abstracted measure sets have been replaced by individual measures and eCQMs have been added to the mix.
So let's review the requirements.
Chart-Abstracted Measure Requirements
Hospitals are required to submit all five available chart-abstracted measures to The Joint Commission. You must continue to submit these measures on a quarterly basis for the entire year.
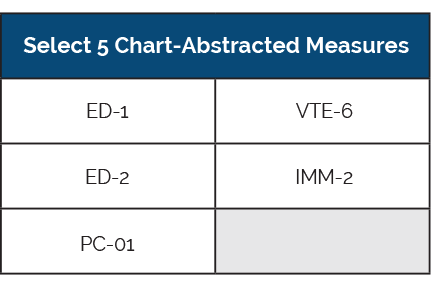
Chart-abstracted Submission Method
Hospitals must use an approved vendor to submit their chart-abstracted measures to The Joint Commission.
Here is The Joint Commission’s list of acceptable vendors. Check us out on page 3!
eCQM Requirements
Originally, The Joint Commission required that hospitals submit six eCQMs, but when CMS released their 2018 IPPS Final Rule in August, the requirements were adjusted. CMS reduced the number of required eCQMs from eight to four for the Inpatient Quality Reporting (IQR) program. The Joint Commission followed suit; reducing the number of required eCQMs from six to four in order to align with CMS. They also lessened the reporting period requirement. Both programs reduced the data submission period from one full year’s worth of data to just one quarter’s worth of data.
Also See: Changes to Your 2017 Reporting Requirements
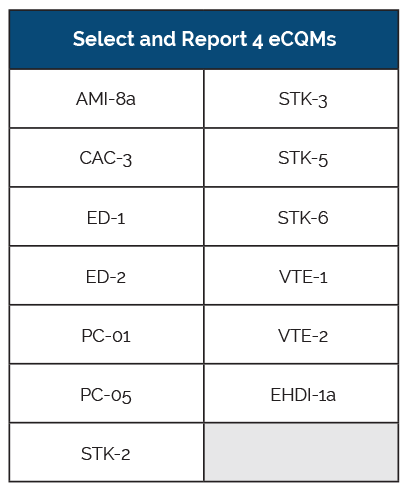
eCQM Submission Method
Unlike with chart-abstracted measure submission, you have two options for reporting your eCQMs.
Option 1: Vendor Submission
The easiest way to submit your eCQM data is by using one of the Joint Commission’s listed vendors that support eCQM submission. For a list of vendors see The Joint Commission’s official list.
P.S. Check us out on page 3. Medisolv is able to submit your chart-abstracted and electronic measures!
Option 2: Direct Submission
You may do a direct submission on your own; however, The Joint Commission doesn’t expect this portal to be open for submission until the start of the new year. And in fact, all they really say is that they will have it available in time for the new June 29th deadline. It will also cost you $300 per hospital per year.
There are a few other requirements you must fulfill if you submit on your own.
- The data file that you submit must use EHR technology that’s certified to the 2014 or 2015 edition of CEHRT.
- The EHR technology must be listed on the ONC Certified Health IT Product List.
- All files submitted to The Joint Commission must be in the QRDA 1 file format.
Special Facility Requirements
Hospitals with at least 300 live births
Hospitals with at least 300 live births per year must meet the requirements listed above, but have additional requirements tacked on. They must also report the following four perinatal care measures.
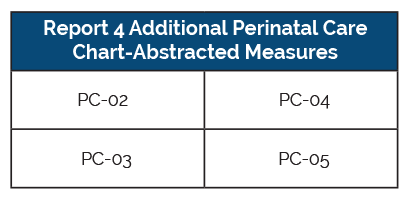
Critical Access and Small Hospitals
Critical Access Hospitals or Small Hospitals (average daily census of 10 or fewer inpatients) are required to report on six measures. These hospitals however may choose to report measures from either the chart-abstracted list or eCQM list or a combination of both (see measures above).
Freestanding Psychiatric Hospitals
Freestanding psychiatric hospitals are only required to report on the HBIPS measures (hospital-based inpatient psychiatric services). Just like the chart-abstracted measure requirements, these facilities must report the data on a quarterly basis for the entire year.
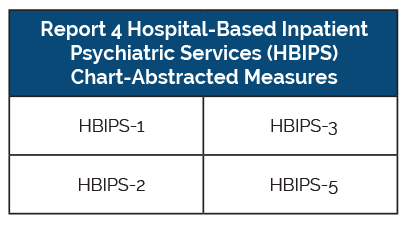
Acute care hospitals with inpatient psychiatric units are not required to report on the HBIPS measures, however, they can choose to report on the HBIPS measures to meet or exceed their requirements.
Facilities with Suspended requirements
The Joint Commission has decided to suspend the ORYX® requirements for the following facilities:
- Freestanding Children’s Hospitals
- Long Term Acute Care Hospitals
- Inpatient Rehabilitation Facilities
There you have it. These are the requirements that are in place for this year and the following year. Don't wait to get started with your reporting to The Joint Commission. Medisolv helps hospitals monitor and submit eCQMs and chart-abstracted measures every year. Our software allows your hospital to align all of your quality improvement efforts from one platform.
So, if you’re interested in learning a bit more about our solution send us a note. We’d love to have a chat and see if we are good fit for your needs.






Add a comment