Questions for our Quality Expert Panel
By Erin Heilman February 2, 2018 Regulatory Updates: Ambulatory,Regulatory Updates: Hospital

Do you have questions about Quality reporting? Well you're not alone. In a recent webinar, Medisolv brought together a panel of Quality experts to answer questions from the audience. Participants submitted all types of questions: big, small, specific, broad, procedural, technical, elementary and advanced.
We've pulled some of the best questions from the webinar. Let's take a look at the answers to these questions. You might just learn something you didn't already know!
Before we begin, let me introduce our panel.

You'll read answers from each of these panel members. Let's begin.
"What is the best way to stay current with specification changes and reporting mandates?"
 The regulatory burden for hospitals is getting quite a bit of attention these days. AHA recently published a paper stating there were 300 regulatory requirements, of which, 50 of them are in the Quality domain. The same paper stated that the average size community hospital dedicates almostfive full-time equivalents (FTEs) directly to Quality reporting. In reaction, CMS said that the current administration is committed to reducing the regulatory burden on hospitals. The administrator at CMS has initiated an effort to review every Quality measure for value. While that process is going on, that leaves everyone else left to read the 11,000 pages that CMS publishes on regulations each year.
The regulatory burden for hospitals is getting quite a bit of attention these days. AHA recently published a paper stating there were 300 regulatory requirements, of which, 50 of them are in the Quality domain. The same paper stated that the average size community hospital dedicates almostfive full-time equivalents (FTEs) directly to Quality reporting. In reaction, CMS said that the current administration is committed to reducing the regulatory burden on hospitals. The administrator at CMS has initiated an effort to review every Quality measure for value. While that process is going on, that leaves everyone else left to read the 11,000 pages that CMS publishes on regulations each year.
So how do you stay on top of all of this? Understand the annual cycle and put a plan in place. Most of this begins with the proposed rule and subsequent final rule. Measures will most likely have specification updates and submission requirements. All of these changes require you to adopt to the new requirements rather quickly. So, below you will see an example of the eCQM annual cycle that we follow here at Medisolv. We use this annual plan for each of our clients to help them stay on top of regulatory updates and submissions each year.
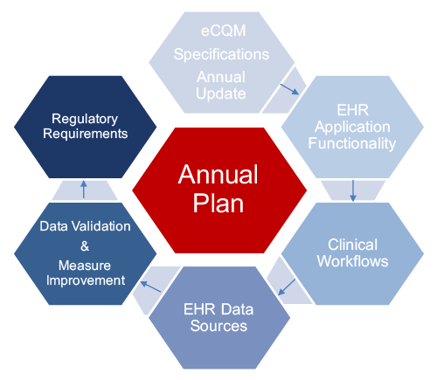
Everything starts with the specifications, which are published in the spring. You may have to adapt clinical workflows or integrate multiple EHR data sources. This all leads you to the data validation and measure improvement process. This process of validation and improvement should be continuous throughout the year so that when it comes time to submit to the regulatory programs you are ready. In a nutshell, plan to put an annual process in place for your hospital regardless of the program or measures you are trying to implement.
"Where do I go to get my EHR Certification ID? Do I need a new one every year?"
 The EHR Certification ID is new for your 2017 eCQM submission to the Inpatient Quality Reporting (IQR) program. You can visit the CHPL website to generate the Certification ID for your EHR.
The EHR Certification ID is new for your 2017 eCQM submission to the Inpatient Quality Reporting (IQR) program. You can visit the CHPL website to generate the Certification ID for your EHR.
Most likely, yes, you will need a new ID every year. If there are any updates or changes to your EHR in the hospital, or certified eCQM reporting solution you would have to go back through the process of updating that information and getting a new ID. So, to be on the safe side, you should get a new ID in place so you are ready for the next submission period.
"What is the difference between the IPP vs Measure Algorithm?"

Initial Patient Population (IPP): The detailed information that describes the population for the measure or indicator you intend to measure. The IPP algorithm evaluates each case for inclusion or exclusion for a measure population.
Measure Algorithm: The measure algorithm assesses each case based upon abstracted data elements and data element questions for exclusion or compliance per the intent of the measure.
"Where on the QPP website can I find the specification sheets?"
 It's actually not as an intuitive as it used to be to find the specification sheet on the QPP or Quality Payment Program website. You must go to the website https://qpp.cms.gov/ and click the word About in the top right corner. In the drop down, click the words Resource Library. On this page you'll see another link called Resource Library to CMS.gov. Believe it or not, this is the convoluted path you must take in order to get to the specification information from the QPP website. Alternatively, you can just click on the link directly to the CMS webpage instead. On this webpage, you will find links to the resources by year or by specialty. Within each year are the specification sheets and any other documentation related to the program.
It's actually not as an intuitive as it used to be to find the specification sheet on the QPP or Quality Payment Program website. You must go to the website https://qpp.cms.gov/ and click the word About in the top right corner. In the drop down, click the words Resource Library. On this page you'll see another link called Resource Library to CMS.gov. Believe it or not, this is the convoluted path you must take in order to get to the specification information from the QPP website. Alternatively, you can just click on the link directly to the CMS webpage instead. On this webpage, you will find links to the resources by year or by specialty. Within each year are the specification sheets and any other documentation related to the program.
"Where do I find measure specifications for PC and HBIPS?"
 You can find measure specifications for these two measures on The Joint Commission's website. The reason why they are housed here is that The Joint Commission is the measure steward for these chart-abstracted measures, not CMS. So if you were looking for these measures, CMS is not the steward.
You can find measure specifications for these two measures on The Joint Commission's website. The reason why they are housed here is that The Joint Commission is the measure steward for these chart-abstracted measures, not CMS. So if you were looking for these measures, CMS is not the steward.
"eCQM Updates: Where do I find the specifications? When can we start mapping and making changes for 2018?"
 The eCQM specifications can be found on the CMS website. There you can find all of the specifications for the current year and years past. The eCQI Resource Center is also very helpful. Not only can you find the measures here, but also a lot of educational materials and checklists.
The eCQM specifications can be found on the CMS website. There you can find all of the specifications for the current year and years past. The eCQI Resource Center is also very helpful. Not only can you find the measures here, but also a lot of educational materials and checklists.
As for making changes, we here at Medisolv start working with our clients in the fall before the new reporting year gets started. This helps our hospitals prepare for the upcoming changes. Generally, you cannot start making any mapping changes until after your previous year's submission. For example, if you were submitting your 2017 eCQM data to the IQR program, you could not make mapping changes until you've submitted that information. So if you don't submit your eCQMs until February of 2018 you can't update your system until after that submission is completed.
You do not want to retroactively change mapping, but you do want to have it in place for the next reporting year. So, even though there may be some overlap, the changes are best to be made at the beginning of the reporting year.
"When will they start publishing our eCQM results? And when will our results impact our reimbursement?"
 CMS has communicated that for calendar year 2017 submissions, no eCQM data will be publicly reported. They are vague about future plans. The only thing to do is to wait for the final rule, which comes out in the fall and communicates whether there will be any published information in 2019.
CMS has communicated that for calendar year 2017 submissions, no eCQM data will be publicly reported. They are vague about future plans. The only thing to do is to wait for the final rule, which comes out in the fall and communicates whether there will be any published information in 2019.
"How do I know that I'm done with submission for CMS?"
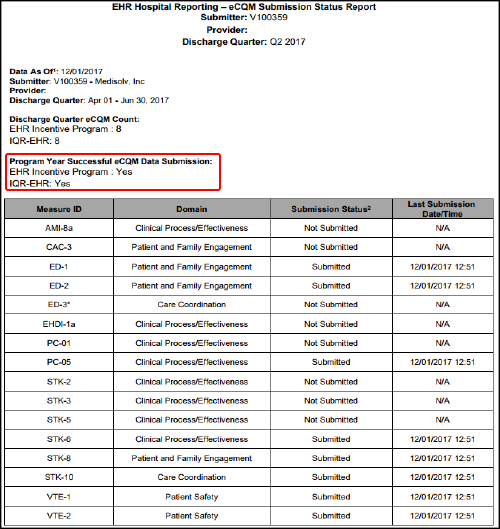
 In theory, this is pretty simple. Below is an example of an eCQM Submission Status Report from CMS. The portion in red under the title, "Program Year Successful eCQM Data Submission" should have marked "yes" for both the EHR Incentive Program and the IQR-EHR.
In theory, this is pretty simple. Below is an example of an eCQM Submission Status Report from CMS. The portion in red under the title, "Program Year Successful eCQM Data Submission" should have marked "yes" for both the EHR Incentive Program and the IQR-EHR.
"What reports should I be running from QualityNet related to my Abstracted submissions?"
 Well, as a vendor, Medisolv may not have access to run the same reports you do as a hospital. With that being said, the CMS reports that we focus on reviewing are the Provider Participation reports, the Population and Sampling reports, Case Status summaries and the Population and Submission report. Keep in mind, running the duplication reports to ensure that the file comes back clean is also important.
Well, as a vendor, Medisolv may not have access to run the same reports you do as a hospital. With that being said, the CMS reports that we focus on reviewing are the Provider Participation reports, the Population and Sampling reports, Case Status summaries and the Population and Submission report. Keep in mind, running the duplication reports to ensure that the file comes back clean is also important.
"Can you talk about the validation process with CMS for eCQMs?"
 We can really only answer based on what has been published; which is that 200 hospitals will be randomly selected for validation or audits "sometime" in the spring of 2018. So audits on eCQMs should be initiated in the upcoming months. All of the documentation indicates that they will request data be electronically submitted from the EHR.
We can really only answer based on what has been published; which is that 200 hospitals will be randomly selected for validation or audits "sometime" in the spring of 2018. So audits on eCQMs should be initiated in the upcoming months. All of the documentation indicates that they will request data be electronically submitted from the EHR.
"How can I find resources related to the chart-abstracted measures?"
 CMS: You can head to the QualityNet website to find specifications related to inpatient, outpatient and HBIPS measures. Here you can also find the specification manuals, which include information related to forums and notes for abstraction.
CMS: You can head to the QualityNet website to find specifications related to inpatient, outpatient and HBIPS measures. Here you can also find the specification manuals, which include information related to forums and notes for abstraction.
The Joint Commission: Under "measurements" on The Joint Commission website, you can find all of the specification manuals for abstracting.
Quality Reporting Center: Here you can find loads of information related to the various programs, including webinars and other educational material.
"What CEHRT will be required for hospitals to receive reimbursement past 2018?"
 CMS has modified the certification requirements for the calendar year 2018 reporting period. Hospitals will be able to continue to use either the 2014 edition of CEHRT, the 2015 edition of CEHRT or a combination of the two, giving some flexibility this year. In 2019, I would definitely plan for the 2015 edition of CEHRT to be a requirement. However, CMS has not clearly stated this will be a requirement in 2019.
CMS has modified the certification requirements for the calendar year 2018 reporting period. Hospitals will be able to continue to use either the 2014 edition of CEHRT, the 2015 edition of CEHRT or a combination of the two, giving some flexibility this year. In 2019, I would definitely plan for the 2015 edition of CEHRT to be a requirement. However, CMS has not clearly stated this will be a requirement in 2019.
"How do you find which Improvement Activity measures are eligible for the Advancing Care Information bonus?"
 All of this information can be found in the same Resource Library on the CMS website. Remember, it's pretty tricky to find it so follow the instructions from the question above. The Improvement Activity measures eligible for the Advancing Care Information bonus are buried in the 2017 resources link in a document titled "Advancing Care Information Performance Category fact sheet."
All of this information can be found in the same Resource Library on the CMS website. Remember, it's pretty tricky to find it so follow the instructions from the question above. The Improvement Activity measures eligible for the Advancing Care Information bonus are buried in the 2017 resources link in a document titled "Advancing Care Information Performance Category fact sheet."
View the Advancing Care Information Performance Category Fact Sheet.
"What are the IPPS requirements for Critical Access Hospitals?"
 On the chart-abstracted side of the world, for Critical Access Hospitals, they are not required to submit the chart-abstracted data to QualityNet. With that being said, Critical Access Hospitals are strongly encouraged to submit their data to allow them to see comparative data and how they stack up against other hospitals.
On the chart-abstracted side of the world, for Critical Access Hospitals, they are not required to submit the chart-abstracted data to QualityNet. With that being said, Critical Access Hospitals are strongly encouraged to submit their data to allow them to see comparative data and how they stack up against other hospitals.
 For the eCQM portion of the IQR program, all of the documentation indicates that Critical Access Hospitals are not required to submit their data electronically. Although, at this point, it makes sense for these hospitals to submit electronically.; if they don't submit eCQMs to the IQR program, then they must attest to all 16 eCQMs for the Meaningful Use program, which could ultimately result in more work.
For the eCQM portion of the IQR program, all of the documentation indicates that Critical Access Hospitals are not required to submit their data electronically. Although, at this point, it makes sense for these hospitals to submit electronically.; if they don't submit eCQMs to the IQR program, then they must attest to all 16 eCQMs for the Meaningful Use program, which could ultimately result in more work.
Is your brain on overload? Are you inspired to ask questions of your own? Good news! Our expert panel is available to answer your questions at any time. If you have a question you would like our expert panel to address, please submit the question to questions@medisolv.com.
Medisolv Can HelpTalk to us today to see a demonstration of our software and learn about the incredible support we give to our clients every day. Here are some additional resources we thought you might find helpful: Blog Post: "Medisolv Quality Expert Panel Answers your FAQ" |






Add a comment