Understanding SAFER Guides and a Review of your 2022 PI Requirements

The Promoting Interoperability (PI) Program originally was known as the Meaningful Use program and got started back in 2009. The program was initiated to get hospitals up and running with their Electronic Health Record (EHR) systems. In 2018, the program got a name change and an overhaul.
Each year there are slight changes. In 2022, there is a new requirement which requires you to attest to whether your organization performed a self-assessment of the nine SAFER guides. To successfully meet the requirement, you must attest to Yes or No. At this point, CMS is just tracking if organizations are using these.
The SAFER Guides
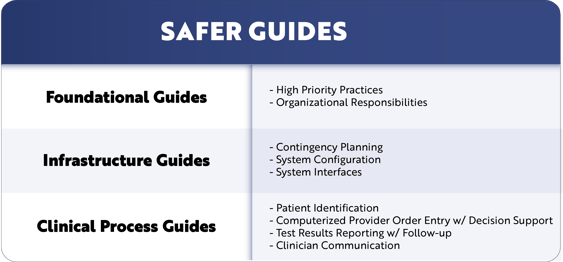
So, what are the nine SAFER guides and where can they be found? The SAFER guides consist of nine guides organized into three broad groups (Foundational Guides, Infrastructure Guides, and Clinical Process Guides). They are designed to help healthcare organizations conduct self-assessments to optimize the safety and safe use of electronic health records (EHRs) in the following areas:
Foundational Guides
Contingency Planning Guides
Clinical Process Guides
- Patient Identification
- Computerized Provider Order Entry with Decision Support
- Test Results Reporting and Follow-up
- Clinician Communication
The SAFER guides are divided up into several sections that can be used for practice implementation. The guides will provide instructions and assessments on the recommended practices as well as implementation suggestions. The content of each SAFER guide can be found on ONC's website. The nice thing about these guides is that they are interactive, can be filled out, saved, and transmitted among your team members.
These SAFER guides were developed by Health IT safety researchers and informatics experts. Each guide starts with a General Instructions and Introduction section where ONC describes the intent of the usage and tracking of these guides. Each Introduction is specific to each guide and explains the potential benefits, recommended practices, and important focuses.
The next sections provide a checklist, team worksheet, and recommended practice worksheets. The items you select on your checklist will automatically update the related sections of the corresponding Recommended Practice Worksheet. Each Recommended Practice Worksheet provides guidance for implementation by giving you the rationale for the practice as well as examples of potentially useful practice scenarios. These worksheets are shareable as well as printable. They also provide suggested sources of input such as clinicians, support staff, clinical administration, EHR developer, and/or Health IT support staff.
These guides are intuitive, easy to understand and use, and provide recommended practices to help you supplement your current safety practices of your EHR. In other words, these guides provide another layer of safety and security to those layers you already have in place as the trustee of the health information of your patients. In an ever-changing healthcare technology world, these are a good resource to use periodically to ensure that you are meeting both the needs of your organization as well as the needs of your patients.
2022 Promoting Interoperability Requirements
Let’s move on to your 2022 PI requirements. Eligible Hospitals and Critical Access Hospitals must meet the following program requirements, or they will be penalized for non-compliance.
Program Reporting Requirements
- Report measures in four categories. You must score 60 points minimum out of 115 total possible points (See PI Reporting Requirements section below)
Other Program Requirements (no points awarded)
- Complete the Security Risk Assessment
- New Submit the Protect Patient Health Information measure (<<these are our SAFER guides)
- Submit your eCQM performance data
Reporting Period
- 90-days
Measure Reporting Requirements
There are four categories of measures you must submit in the PI program. CMS calls these Objectives. You must report certain measures within each category (outlined below). Based on what you submit for these measures you will be awarded points. You must score a minimum of 60 points to satisfy your PI requirements.
Public Health and Clinical Data Exchange Category
Requirement:
- You must report “yes” for all 4 measures in this category or take an applicable exclusion. If you can claim Active Engagement that will still count as meeting the measure. (possible 10 points)
- Syndromic Surveillance Reporting
- Immunization Registry Reporting
- Electronic Case Reporting
- Electronic Reportable Laboratory Results
Bonus:
- Report 1 of the following measures (worth 5 bonus points)
- Public Health Registry Reporting
- Clinical Data Registry Reporting
Exclusions:
- If you claim an exclusion on 1-3 measures in this category you can still receive 10 points. You must report a “yes” response to any of the measures you did not claim an exclusion on.
- If you claim an exclusion on all 4 measures in this category your points are redistributed to the Provider to Patient Exchange category.
- If you don’t report on any 1 of these measures OR report “no” you will receive a score of zero AND a total score of zero for the entire Promoting Interoperability program.
Electronic Prescribing (ePrescribing) Category
Requirement:
- You must report this 1 measure in this category (possible 10 points)
- ePrescribing
Bonus:
- Report this measure (worth 10 bonus points)
- Query Prescription Drug Monitoring Program (PDMP)
Provider to Patient Exchange Category
Requirement:
- You must report this 1 measure in this category (possible 40 points)
- Provide Patients Electronic Access to Their Health Information
Health Information Exchange Category
Requirement:
- There are two options for reporting this category in 2022. The first option is the same as previous years with two separate measures. The second option is brand new and is only one measure.
Option 1:
- Report on both measures below. You must have a “1” in the numerator for both measures (possible 20 points for each measure – total 40 points)
- Support Electronic Referral Loops by Sending Health Information
- Support Electronic Referral Loops by Receiving and Reconciling Health Information
Option 2:
- Report on this one measure. This measure does not require a numerator or denominator. You must attest to a “Yes” response. (possible 40 points)
- Health Information Exchange Bi-Directional Exchange
Measure Detail:
For Option 2, you must attest that you engage in bi-directional exchange with a Health Information Exchange (HIE) to support transitions of care. You are attesting to the following statements:
-
- You participate in an HIE to enable secure, bi-directional exchange of information to occur for all unique patients admitted to or discharged from the hospital inpatient or emergency department (POS 21 or 23), and all unique patient records stored or maintained in the EHR for these departments, during the EHR reporting period in accordance with applicable law and policy.
- You participate in an HIE that is capable of exchanging information across a broad network of unaffiliated exchange partners including those using disparate EHRs, and not engaging in exclusionary behavior when determining exchange partners.
- Using the functions of CEHRT to support bi-directional exchange with an HIE.
Other Program Requirements (no points awarded)
Security Risk Analysis
Requirement:
- You must conduct an annual security risk assessment and attest that it has been completed.
Protect Patient Health Information
Requirement:
- This is the SAFER guides we talked about earlier. Remember that you can attest 'Yes' or 'No' without getting a penalty.
- The measure is asking if your organization has conducted an annual self-assessment of all nine SAFER Guides (outlined below). This assessment had to have taken place during the calendar year of your reporting year (so it doesn’t have to be done in that narrow 90-day timeframe).
SAFER Guides:
Submit your eCQM performance data
Requirements:
- You must submit 4 eCQMs with 3 quarters of data. If you participate in the IQR program and submit your eCQMs for that program, you do not need to submit them again.

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments