Hospital IQR Program Reporting Requirements for 2017

* NOTE Since this blog was written, CMS has updated their eCQM submission deadline for 2017. The deadline is now March 16, 2018.
It's summertime! Are you ready to bust out the sunscreen, grab your sunglasses and mosey down to the nearest beach? Or do you find yourself up to your ears in work? With the middle of the year upon us, are you running from meeting to meeting leaving with a task list that grows longer with each day? I am going to guess you may fall into the latter of these two descriptions.
To help you out, in this article we've compiled the requirements for the CMS Inpatient Quality Reporting (IQR) program to hopefully make your life just a little bit easier. We've included an easy to read summary of the requirements for the program as well as all of the 2017 deadlines.
To successfully report to the CMS IQR program, Eligible Hospitals must submit the following measures.
2017 IQR Program Reporting Requirements
REPORT 6 Chart-Abstracted Measures
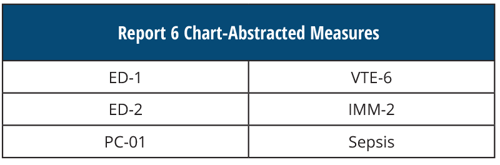
[AND]
SELECT 4 Electronic Clinical Quality Measures (eCQMs)
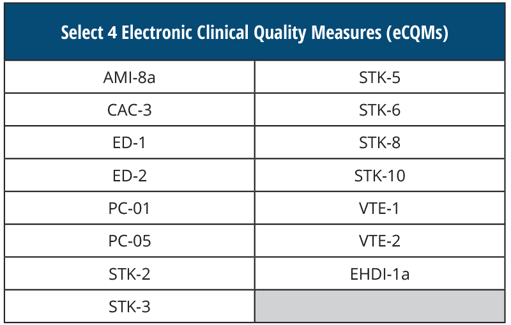
By submitting these eCQMs, you will fulfill your CQM requirements for the EHR Incentive Program, otherwise known as the Meaningful Use program.
[AND]
REPORT 6 NHSN Measures
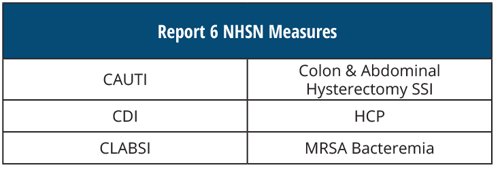
[AND]
REPORT 1 Patient Experience of Care Survey Measure

[AND]
REPORT 2 Structural Measures
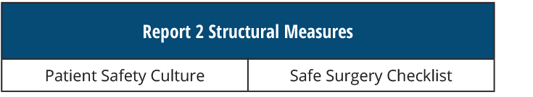
[AND]
REPORT 20 Claims-Based Outcome Measures
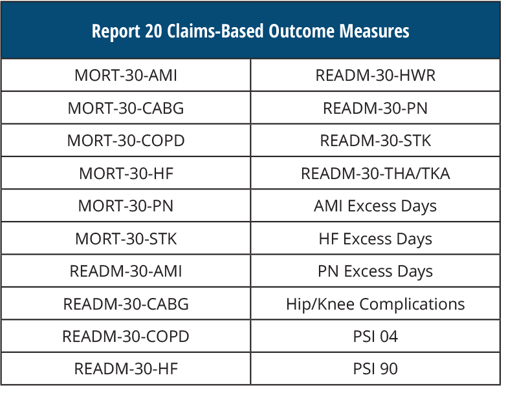
[AND]
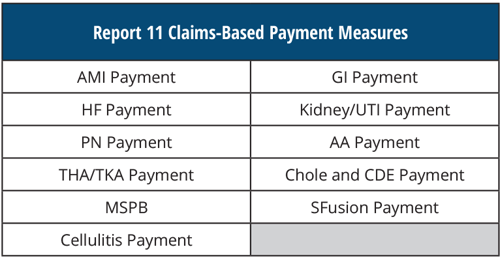
REPORT 11 Claims-Based Payment Measures
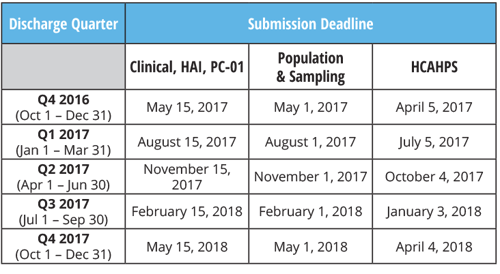
2017 IQR Program Reporting Deadlines
Clinical, HAI, PC-01: Quarterly Submission
Population & Sampling for Clinical: Quarterly Submission
HCAHPS: Quarterly Submission
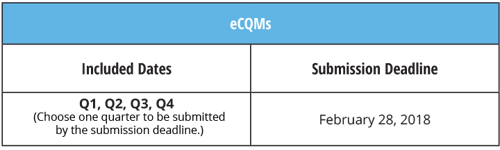
eCQMs: Submit quarterly information by the February 28, 2018 deadline.
Keep in mind again that this was also proposed to be modified in the recent rule.They are proposing to cut it from requiring you to submit all four quarters of data down to just any two quarters of data. The submission deadline remains the same. Again, we suggest that you act as though you will need to submit all four quarters. Bonus, if you submit two quarters early on, then you'll be done for the year if the proposed rule becomes finalized.
Structural: Reporting between April 1 – May 15, 2018
DACA: Reporting between April 1 – May 15, 2018
Influenza Vaccination Coverage Among HCP: May 15, 2018

Your data must be submitted no later than 11:59 p.m. PT on the submission deadline with the exception of HCAHPS, which must be submitted by 11:59 p.m. CT; validation medical records must be received by CDAC no later than 4:30 p.m. ET. Validation for fiscal year 2019 includes Q3 2016, Q4 2016, Q1 2017, and Q2 2017.
Hospitals with Modified Requirements
There are many different circumstances that would qualify a hospital to have either modified requirements or to be excluded from the IQR program altogether. We’ve included a link here to the 2017 IPPS Final Rule starting with the section on excluded hospitals.
However, the best way to understand your requirements is to speak with a Medisolv representative who can walk you through your hospital’s specific needs.
Medisolv Email: questions@medisolv.com
Medisolv Phone: (844) 633-4765
Download:
The 2018 quality reporting bundle
Successfully reporting your eCQM and chart-abstracted measure data to CMS and The Joint Commission is a process that requires some time and preparation. But how can you prepare without the proper information? Do you know what to search for? Is the information that you found correct and accurate for this submission year?
Set your worries aside. We know that you have more important things to do than dig through a handful of confusing CMS and Joint Commission documents—that’s why we did it for you! Yup, true story. We gathered all of the important documents, standardized and simplified them and put everything in our 2018 Quality Reporting Bundle.
Woot woot! *Pause for dance party and sigh of relief.*
Check out what you’ll find in this handy bundle below.
CMS IQR Program:
- Reporting requirements
- Measure list
- Deadlines
The Joint Commission ORYX® Initiative for Quality Improvement:
- Reporting requirements
- Measure list
- Deadlines
Oh, AND an Acronym Reference Guide. No more guessing game.





.png?width=352&name=blogimage_A%20Review%20of%20Hybrid%20Measure%20Changes%202024%20%E2%80%93%202027%20(1).png)
Comments