2019 eCQM Requirements for the CMS IQR Program

The requirements released in the 2019 IPPS Final Rule have since been updated in response to COVID-19. To learn more about these changes, read our blog on quality reporting changes due to COVID-19.
CMS requires eCQMs to be submitted as a part of successfully completing your Inpatient Quality Reporting (IQR) program requirements. In this post, we review the 2019 eCQM requirements for the IQR program. Watch the video below for a quick overview.
ALSO SEE: 2019 eCQM requirements for The Joint Commission ORYX® initiative for quality improvement program.
Reporting Requirements
Just as in 2018, hospitals must continue to submit four of the available 15 eCQMs to the program.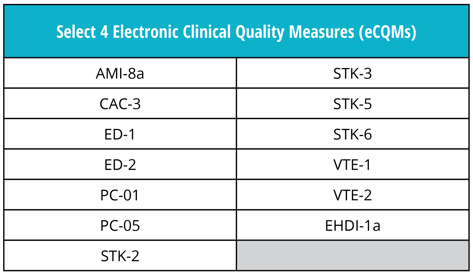
Remember, eCQM submissions to the CMS IQR program are separate from eCQM submissions to other major regulatory program such as the The Joint Commission ORYX® initiative for quality improvement program.
When submitting data, hospitals must use the most recent version of the eCQM specifications which, as of this post, is May of 2018. Your data also must be submitted using the QRDA (Quality Reporting Document Architecture) Category 1 file format.
Unlike in 2018, your EHR must be certified to the 2015 edition of Certified EHR Technology (CEHRT) for reporting. You will no longer be able to use the 2014 Edition of CEHRT.
Reporting Deadline:
In response to COVID-19, CMS has made the following changes:
- Q4 data submission is optional.
- If you do submit, CMS will use your entire year of data including Q4 to calculate your payment as applicable to each part of the program.
- If you don’t submit, CMS will use the data you submitted for January 1 – September 30 to calculate your performance and payment (where appropriate).
Just as in 2018, you can choose to report data from any one quarter of 2019. The submission window opens this summer, so we suggest that you submit your measures as soon as possible instead of rushing to get it done at the last minute. However, you do have until February 29, 2020 to get your data submitted to CMS and since February 29 falls on a Saturday next year, you technically have until March 2, 2020 to submit your data.
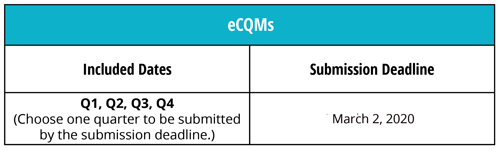
Note: Your data must be submitted no later than 11:59 p.m. PT by the submission deadline date.
Additional Guidelines:
In order to achieve a successful eCQM submission, there are a few guidelines that you need to follow.
- The data file that you submit must use EHR technology, like Medisolv, that’s certified to the 2015 edition of CEHRT.
- Vendors must be certified for all 15 eCQMs regardless of which eCQMs you submit.
- You must use the latest version of the specifications, which is currently May of 2018.
- All files submitted to CMS must be in the QRDA 1 file format.
Medisolv offers a quality reporting solution that helps hospitals and clinicians monitor and submit their eCQMs to various regulatory programs such as The Joint Commission and CMS. We also have dedicated clinical experts who can help with implementation, validation and submission of eCQMs.
Looking for eCQM assistance? Send us a note. We’d love to have a chat and see if we are a good fit for you.
On-Demand Webinar Series:
ECQM SPRING TRAINING
Get full access to our eCQM Spring Training webinar series on-demand! This series provides a complete understanding of eCQMs, how to implement and report these measures. Each video recording is only 30 minutes.
We coach you through every step of your eCQM spring training journey. We provide you with the tips, tools and resources needed to achieve your eCQM goals and confidently cross the submission finish line.
ON-DEMAND SESSIONS INCLUDE
- Laying the Groundwork: Understanding eCQM basics (specifications, logic).
- Setting your eCQM Training Schedule: Implementing eCQMs and creating a sustainable maintenance plan.
- eCQM Stumbling Blocks Part 1: Common errors in clinical documentation and workflows.
- eCQM Stumbling Blocks Part 2: Common errors in timing, logic and mapping.
- Ready, Set, Go: Running the Race: Reviewing the 2019 eCQM regulatory requirements and the submission and post-submission review process.


.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments