2021 Joint Commission ORYX Requirements

There are some big changes for The Joint Commission’s ORYX® program in 2021. To be honest, the biggest challenge we foresee is keeping the requirements straight between the ORYX® program and the CMS Inpatient Quality Reporting (IQR) program. They used to be mostly identical and now they are similar but with enough changes to confuse us all.
Let’s review some of the major changes to the ORYX® program and then go over the actual requirements for 2021.
TJC Change #1: eCQM changes
Just like the CMS IQR program, there are some big changes to the eCQM portion of the ORYX® program.
-
You must submit two quarters of data instead of one (same as IQR).
-
You have to submit the same eCQMs for both quarters (unclear yet for IQR).
-
There are two new eCQMs available - Safe Use of Opioids - Concurrent Prescribing (same as IQR) and a brand new eCQM ePC-06 (not available in the IQR program).
-
Instead of submitting the required PC abstracted measures you can submit the eCQM equivalent for two quarters.
-
As of now, TJC will not publicly report eCQM results (IQR eCQM results will be published by CMS).
TJC Change #2: Chart-abstracted measure changes
In 2021, the abstracted measure list is centered on perinatal care. If your hospital has 300 or more live births you must submit PC-01, PC-02, PC-05 and PC-06. If you have 1-299 live births you must only submit PC-01, otherwise no submission is required.
Additionally:
- The quarterly deadline dates are changed to three months after the end of each quarter. (They used to be four months after the end of each quarter. The new dates are 6/30/2021, 9/30/2021, 12/31/2021 and 03/31/2022.)
- TJC has eliminated the “self-reporting” option in 2021. Everyone must submit to the Direct Data Submission Platform (DDSP).
- TJC has eliminated the ORYX® Designated specialty hospital title. Going forward hospital’s requirements will be based upon licensed beds and/or outpatient visits.
TJC Change #3: Critical Access Hospital changes
If you are a CAH you are now required to report data. In prior years it was voluntary but now you are required - although your requirements are modified (see requirements below).
That covers the major changes you should be aware of. Now let’s move onto the requirements for the ORYX® program in 2021.
2021 TJC ORYX® program requirements
If you want it, here is The Joint Commission’s PDF of 2021 requirements for reference.
Chart-abstracted measure requirements
The Joint Commission changed how they categorize hospitals, but the requirements essentially remain the same. Remember that Critical Access Hospitals are now required to submit data (abstracted and/or eCQMs) and everyone must submit using the DDS platform. They also changed the deadlines for the abstracted measures (see below).
Note: For any or all of the required chart-abstracted Perinatal Care Measures (PC-01, PC-02, PC-05 and PC-06), hospitals may submit a minimum of two quarters of eCQM data instead of four quarters of the corresponding chart-abstracted measures.
Requirement:
|
Chart-Abstracted Measures |
|||||
|
Hospitals with ≥26 beds OR ≥50,000 Outpatient visits AND: 300+ live births |
Hospitals with ≥26 beds OR ≥50,000 Outpatient visits AND: 1-299 live births |
Hospitals with ≥26 beds OR ≥50,000 Outpatient visits AND: No OB services |
Freestanding Psychiatric Hospitals | Critical Access Hospitals | Hospitals with <26 beds AND <50,000 Outpatient visits |
| PC-01 PC-02 PC-05 PC-06 |
PC-01 | None | HBIPS-1 HBIPS-2 HBIPS-3 HBIPS-5 |
Submit any combination of 3 eCQMs and/or chart-abstracted measures | Submit any combination of 3 eCQMs and/or chart-abstracted measures |
Deadlines:
Q1 due 6/30/2021
Q2 due 9/30/2021
Q3 due 12/31/2021
Q4 due 03/31/2022
eCQM requirements
In 2021, hospitals must submit two quarters of data instead of one quarter. The eCQMs must be the same for both quarters. TJC added two new eCQMs, Unexpected Complications in Term Newborns (ePC-06) and Safe Use of Opioids - Concurrent Prescribing (eOPI-1). Remember that this data must still be submitted via the DDS platform, but unlike the abstracted measures, eCQMs must still contain patient-level data in the form of a QRDA I file format. Also, as of now, TJC will not publicly report eCQM results unlike CMS who will publish the IQR eCQM results.
Note: For any or all of the required chart-abstracted Perinatal Care Measures (PC-01, PC-02, PC-05 and PC-06), hospitals may submit a minimum of two quarters of eCQM data instead of four quarters of the corresponding chart-abstracted measures.
Requirement:
|
eCQMs |
|||||
|
Hospitals with ≥26 beds OR ≥50,000 Outpatient visits AND: 300+ live births |
Hospitals with ≥26 beds OR ≥50,000 Outpatient visits AND: 1-299 live births |
Hospitals with ≥26 beds OR ≥50,000 Outpatient visits AND: No OB services |
Freestanding Psychiatric Hospitals | Critical Access Hospitals | Hospitals with <26 beds AND <50,000 Outpatient visits |
| Submit 4 eCQMs, reporting the same eCQMs for 2 self-selected quarters | Submit 4 eCQMs, reporting the same eCQMs for 2 self-selected quarters | Submit 4 eCQMs, reporting the same eCQMs for 2 self-selected quarters | HBIPS-1 HBIPS-2 HBIPS-3 HBIPS-5 |
Submit any combination of 3 eCQMs and/or chart-abstracted measures | Submit any combination of 3 eCQMs and/or chart-abstracted measures |
Annual Deadline:
March 15, 2022
Facilities with suspended requirements
As in the past, the following facilities are exempt from The Joint Commission ORYX® requirements in 2021:
- Freestanding Children’s Hospitals
- Long Term Acute Care Hospitals
- Inpatient Rehabilitation Facilities
- Hospitals participating in the CMS PPS-Exempt-Cancer Hospital Quality Reporting program
CMS vs TJC measure lists
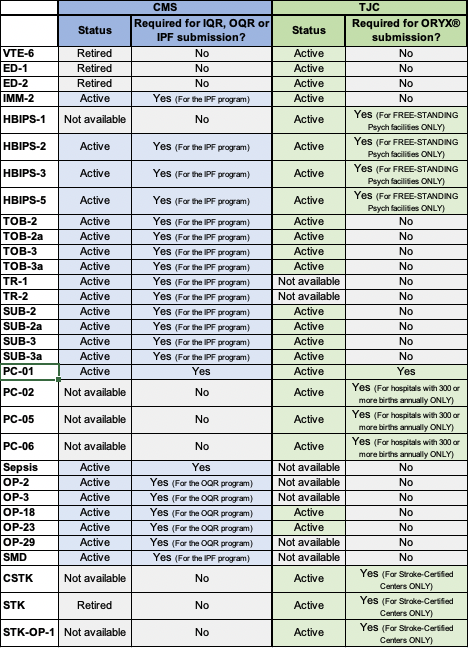
Usually we have found that TJC tries to align their measure lists with the CMS programs, but over the course of the last few years things have diverged. So, to provide a comparison for you, we’ve laid out the measure lists side-by-side so you can identify where the differences lie.
This was such a pain to pull together, so I made it into a downloadable Excel workbook for you to have. The download button is below.
Beginning with January 1, 2021.
| Download these reference charts for excel: | |
2021 TJC vs CMS Inpatient Chart-Abstracted Measure List
2021 TJC vs CMS Inpatient eCQM List
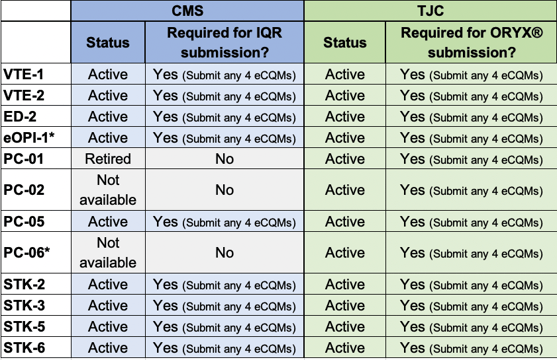
* New eCQM for 2021.
Note: The Safe Use of Opioids eCQM (eOPI-1) is available in both programs next year and CMS has mandated submission of this eCQM for 2022.
2021 is a year of significant change for CMS and The Joint Commission and Medisolv can be your partner through this transition. It’s not enough to just submit your measures, things are starting to heat up and if you don’t have someone there to keep you updated on changing measure requirements and regulatory expectations you may fall behind.
If you are using another vendor or your EHR, ask them when they will have these new eCQMs ready to track and monitor. Or ask them about their plan for the new Hybrid measure. Medisolv is already helping our clients monitor and improve their performance on all of these new electronic measures.
Medisolv Can HelpTalk to us today to see a demonstration of our software and learn about the incredible support we give to our clients every day. In the meantime, here are some resources that will help you get started. On-Demand Webinar: eCQM 101: Getting Started with Electronic Measurement |

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments