Preparing for Regulatory Requirement Changes Using An Annual Cycle

Over the last few months there have been a few changes to Quality reporting regulatory programs. The 2018 IPPS Final Rule overrode the requirements from the 2017 IPPS Final Rule. It makes you wonder, "Is anything ever final in quality reporting?"
Also see: Changes to Your 2017 eCQM Reporting Requirements
With the uncertainty surrounding Quality reporting, it's important that you remain a pillar of consistency in shifting sands. In a recent Medisolv survey, respondents indicated that over the last five years, their job has become harder. And the common reason for job complication is the increased volume of regulatory programs.
The eCQM Annual Cycle
Over the years, Medisolv has developed an Annual Cycle to assist hospitals in preparing for submission to these programs. In fact, even with the change this year, the majority of our hospitals have already completed their eCQM requirements for the Inpatient Quality Report (IQR) program by following this methodology.
In this blog we use the eCQM requirements as a model for the Annual Cycle. We encourage you to review the cycle and find ways to apply this model to all of the programs in which you participate. This model works very well. It's a lot of work initially, but once the process is in place it allows hospitals to more easily manage their eCQMs and ultimately improve their results, which is the end goal.
Phase 1: Education & Planning
The first step is to understand and define your eCQM goals. Are you going to report for just one quarter or will you submit more than one quarter? What are the regulatory requirements for the program?
In phase 1 you will also need to set up a good team with a clear process. This team will be responsible for monitoring and managing changes to eCQMs at your facility year after year.
It's not just the reporting requirements that change. The eCQM specifications (critical to eCQM capture) are updated annually.
Ensure you understand the specification logic because it can be confusing if you haven't looked at it before. This is where all of your eCQM results come from. Evaluating the changes to the specifications and the value sets can be very time and resource consuming, which is why it's critical for you to have all hands on deck so your team can quickly implement those changes.
It's best to lay out a clear process for the team to follow. Ensure that you have regularly scheduled meetings to talk about the updates and what the ramifications will be. Make sure everybody understands how those changes are going to impact them and impact their reporting. Review reporting requirements, specification changes, measure logic and value set updates with your team.
Phase 2: Discovery & Build
The next phase involves undergoing a current state assessment and then building out the necessary changes to facilitate performance improvement.
After you've set your goals and reviewed the updates, it's time to assess where you currently stand. What functionalities do you have in place in your EHR? What don't you have in place? Look at your clinical workflows and compare those to where the eCQM is pulling the data. What workflow changes do you need to make in order to capture the data appropriately? What needs to be mapped?
You also want to consider EHR updates. Are you moving to a new EHR? Do you have updates that are coming in? Do you have new functionality that is going to be in place?
Annually, take a step back and look at all of these items, especially as those new specifications are implemented. Perhaps you've decided to implement a new measure next year. If so, you should comb through the measure details and discover how you are currently capturing that data and determine the best way to build what you need to meet that measure. Then move forward by thoughtfully building those updates in your system. Ensure it's a process that works well for both the IT team and the clinicians.
Review the data. Complete a lot of research. Ask a lot of questions. Revise the processes. Test. Repeat.
This phase gets repeated throughout the year. A Quality professional may find that something is not being documented as it should be. The IT team then, needs to get involved to do some more builds and readjust the process.
Of course once you've built the changes you must concurrently train hospital staff. Every update that changes clinical workflow will require additional education.
Don't just tell your staff they have to do it. Explain to your clinicians the reason why they need to click one more button.
Phase 3: Evaluation
Once you get through those two major hurdles the process becomes more refined. If your data is mapped properly, it's time to begin evaluating or validating your data.
Make sure you have a good reporting tool (like Medisolv's ENCOR software) that allows you to easily see your data on a regular basis. Drill down to the patient-level data within your reports in order to understand what was captured and what's missing. Why did the patient fail the measure? Was it a technical error such as mapping or timing? Was it a compliance issue?
With our hospitals, we go through an initial validation phase where we just want to make sure that they are getting something in the reports. Then we move to an advanced level where we perform an in-depth evaluation. The Quality team analyzes the data and IT supports that analysis.
Again, your team will play a critical role in this phase. Ideally, in your regularly scheduled meetings, Quality will present gaps they have noticed in the data. IT will then work on addressing those gaps from the technical side while Quality addresses any compliance issues with the clinicians.
Also see: eCQM Data Validation: Practices From Our Most Successful Hospitals
This process continues for the majority of the year. Throughout this phase you want to be thinking about which eCQMs you are going to submit to the reporting programs. Have an idea of where you're going to have good numbers and then track and improve those numbers. This ensures that you will be confident in the eCQM data that you submit to CMS and The Joint Commission.
Phase 4: Submission
For most of our hospitals these days, submission is the easy part. Everything leading up to this phase is where the difficult work takes place.
Here is an example of what a successful eCQM submission to CMS looks like.
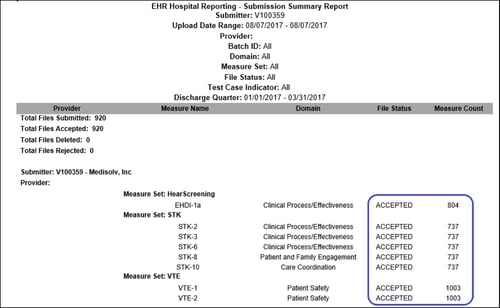
This is what you want to see after you submit to Quality Net. Notice that all files are marked as accepted on the submission summary report.
Unfortunately, the process doesn't end with submission. We recommend a post-submission validation as well. Using the eCQM Performance Summary Report below, compare what this report says the results were versus what the results were in your eCQM reporting tool. Make sure those results align. If not, work on addressing any gaps in performance.
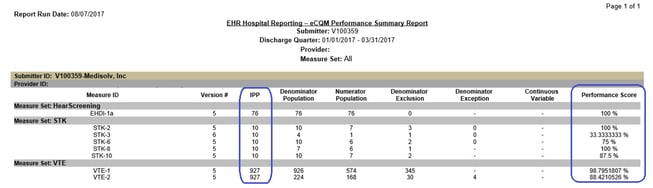
It's important to have this process, or something similar, in place at your facility. With the many changes happening in the healthcare space, this methodology allows your team to account for changes and succeed even when CMS makes a significant change at the last minute.
Remember that it's a group effort between the staff at your facility, your reporting vendor and your EHR vendor. If you would like to talk with us about getting your team on track for successful reporting across all Quality programs, shoot us an email. We'd love to talk to you about how we can help your team.

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments