Super IQR World: Deadlines for the 2019 IQR Program

Major deadlines for reporting to the CMS Inpatient Quality Reporting (IQR) program are rapidly approaching, but it's not game over just yet! Power-up your education by transporting into the Super IQR World.
Navigate through pipe portals, underground dungeons, lava and open water to avoid gaps in your submissions. What obstacles will you encounter on your way to the Castle of Submission Success? Will you earn some cash (or gold coins) on your journey? Or will you be defeated by the big, bad CMS boss (don't tell CMS we said that). There’s only one way to find out— grab your red hat and let's get started!
-
Requirement change:
Q4 data submission is optional. -
Abstracted measures
Q4 data is optional to submit in 2020. -
Population & sample size counts
Q4 data is optional to submit in 2020. -
eCQMs
No requirement changes.
If you were unable to meet the March 2, 2020 deadline you may submit an ECE form by May 1, 2020. -
HAI measures
Q4 data is optional to submit in 2020. -
HCP Influenza Vaccination (HCP)
You are not required to report this data in 2020. -
HCAHPS survey
Q4 data is optional to submit in 2020. -
DACA submission
You are not required to submit the 2019 DACA in 2020. -
Claims data
Q4 data will not be considered. -
Audits
Q3 & Q4 abstracted measure audits do not need to be submitted.
Q4 HAI audits do not need to be submitted.
Any 2019 eCQM audit requests do not need to be submitted.
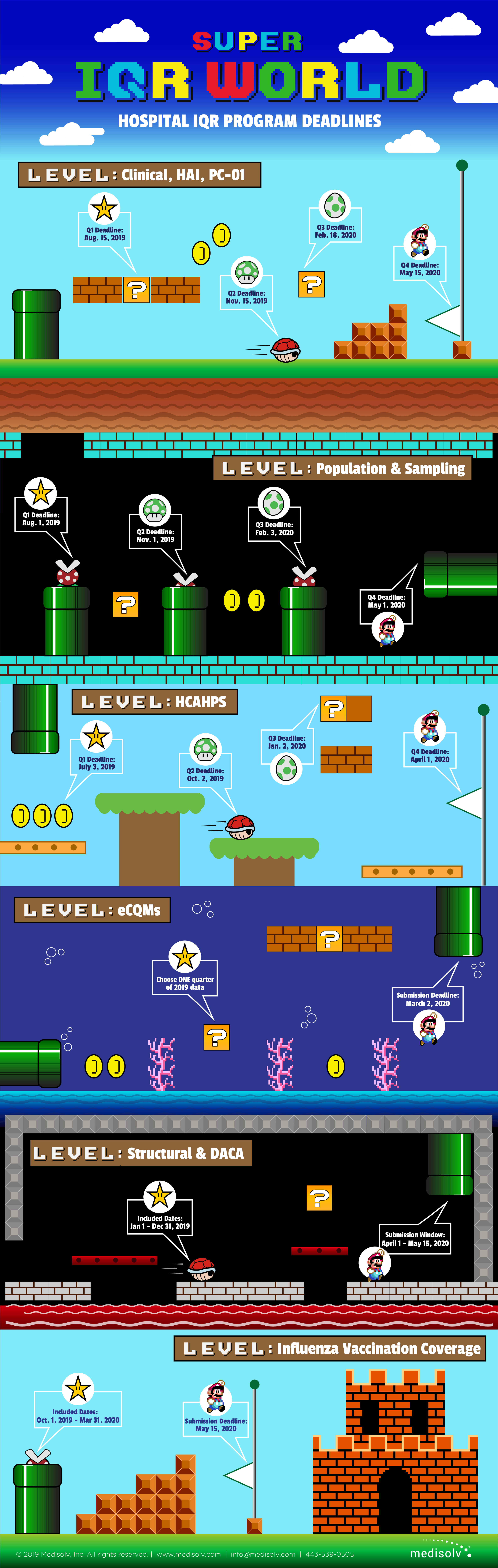
*All dates are subject to change.
You made it!
Now you can download this poster, hang it on your wall, put on a pair of 80s retro sunglasses and people might mistake you for a hipster. Just don’t let them see you pouring over the latest CMS ruling.
If you don’t want to let your retro-self fly, you can download this very sensible calendar which has all of the dates and deadlines laid out in neat calendar months. Then you can go sharpen your pencils…


.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments