IQR Measure Removal: How Will It Affect Your 2019 Reporting Process?

Measures and requirements for the major regulatory programs are continually changing—and next year is no exception.
If you read through the 2019 IPPS final rule that was published this summer (without falling asleep…wait, what?), you learned that CMS is striving to reduce the regulatory reporting burden by removing measures from the Hospital Inpatient Quality Reporting (IQR) program. In fact, for 2019, they adopted a new measure removal factor (justification for removing a measure) that helps boost the count of measures removed from the IQR program to 39 eliminated measures between 2019 and 2021.
So, how will these removed measures ease your hospital’s IQR duties in 2019? This week we’re reviewing the measures that will be removed and categorizing those measures into small, medium and large burden reduction buckets.
First, let’s review the measure removal factors.
CMS measure removal factors
Historically, CMS has used seven factors to determine whether or not a measure should be removed:
- The measure is topped out
- The measure does not align with the current clinical guidelines or practice
- There is availability for a more broadly applicable measure
- Performance or improvement on the measure does not result in better patient outcomes
- The availability of a measure that is more strongly associated with desired patient outcomes for the particular topic
- Collection or public reporting of the measure leads to negative, unintended consequences other than patient harm
- It is not feasible to implement the measure specifications
And in 2019, CMS is implementing an eighth factor for measure removal:
8. The costs associated with a measure outweigh the benefits of its continued use in the program
Three Categories of IQR Measure BUrden Reduction
As I mentioned before, there are quite a few measures that are being removed in 2019. These measures can be categorized into three buckets of burden reduction: significant burden reduction, medium burden reduction and minimal burden reduction. We are going to take a look at which measures will ease your burden, and by how much, as classified by these categories.
In the charts below, we’ve included all of the measures that are currently a part of the IQR program to help you visualize what will be removed and when.
Significant Burden Reduction:
In this category, you can expect to have your reporting burden significantly reduced. Here, CMS removed measures where the collection and submission of quality measures data was required.
Chart-Abstracted Measures
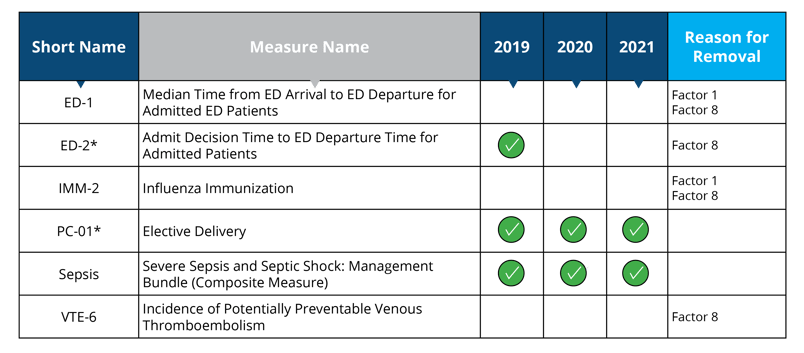
Measures removed starting in 2019: ED-1, IMM-2, VTE-6
Measures removed starting in 2020: ED-2
Measure removal factor 1 and 8:
- Topped out (ED-1 and IMM-2)
- The cost associated with the measures outweigh the benefits of their continued use in the program (ED-1, IMM-2 and VTE-6)
- The costs associated with this measure outweigh the benefit of its continued use; eCQM version of the measure will remain in the Hospital Inpatient Quality Reporting Program (ED-2)
eCQMS
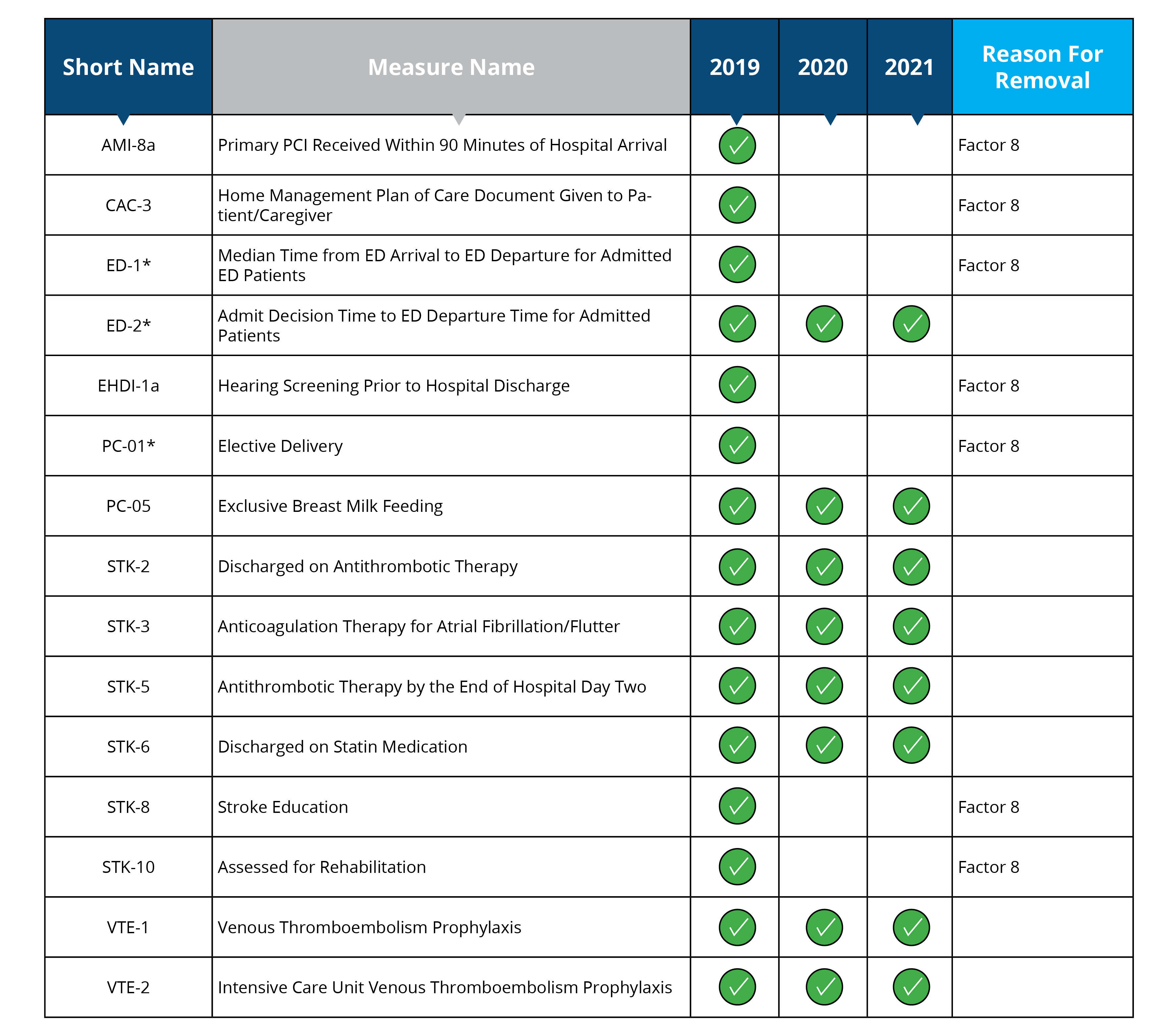
Measures removed starting in 2020: AMI-8a, CAC-3, ED-1, EHDI-1a, PC-01, STK-8, STK-10
Measure removal factor 8:
- The costs associated with the measures outweigh the benefits of their continued use in the program (AMI-8a, CAC-3, ED-1, EHDI-1a, PC-01, STK-8 and STK-10)
Structural Measures
Measures removed starting in 2019: Patient Safety Culture and Safe Surgery List
Measure removal factor 4 and 8:
- Performance or improvement on the measure does not result in better patient outcomes (Patient Safety Culture)
- The cost associated with the measures outweigh the benefits of their continued use in the program (Safe Surgery List)

Medium Burden Reduction:

In this category, you can expect to have your reporting burden moderately reduced. For these measures, no special data collection or submission of quality measures data was required even though you still would have had to review the measures throughout the year and work toward performance improvement.
Claims-Based Measures
Claims-Based Mortality Outcome
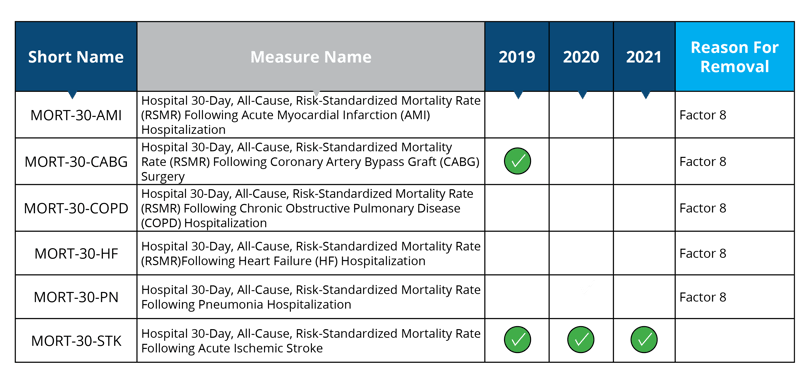
Measures removed starting in 2019: MORT-30-AMI, MORT-30-HF, MORT-30-COPD, MORT-30-PN
Measure removed starting in 2020: MORT-30-CABG
Measure removal factor 8:
- The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP (MORT-30-AMI, MORT-30-CABG, MORT-30-HF, MORT-30-COPD and MORT-30-PN will remain in the HVBP program)
Claims-Based Patient Safety
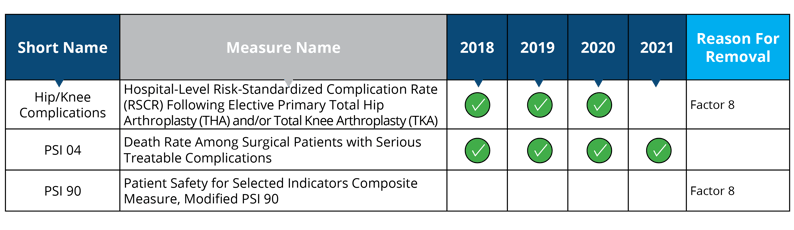
Measure removed starting in 2018: PSI 90
Measure removed starting in 2021: Hip/Knee Complications
Measure removal factor 8:
- The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP (PSI 90 will remain in the HACRP program)
- The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP (Hip/Knee Complications will remain in the HVBP program)
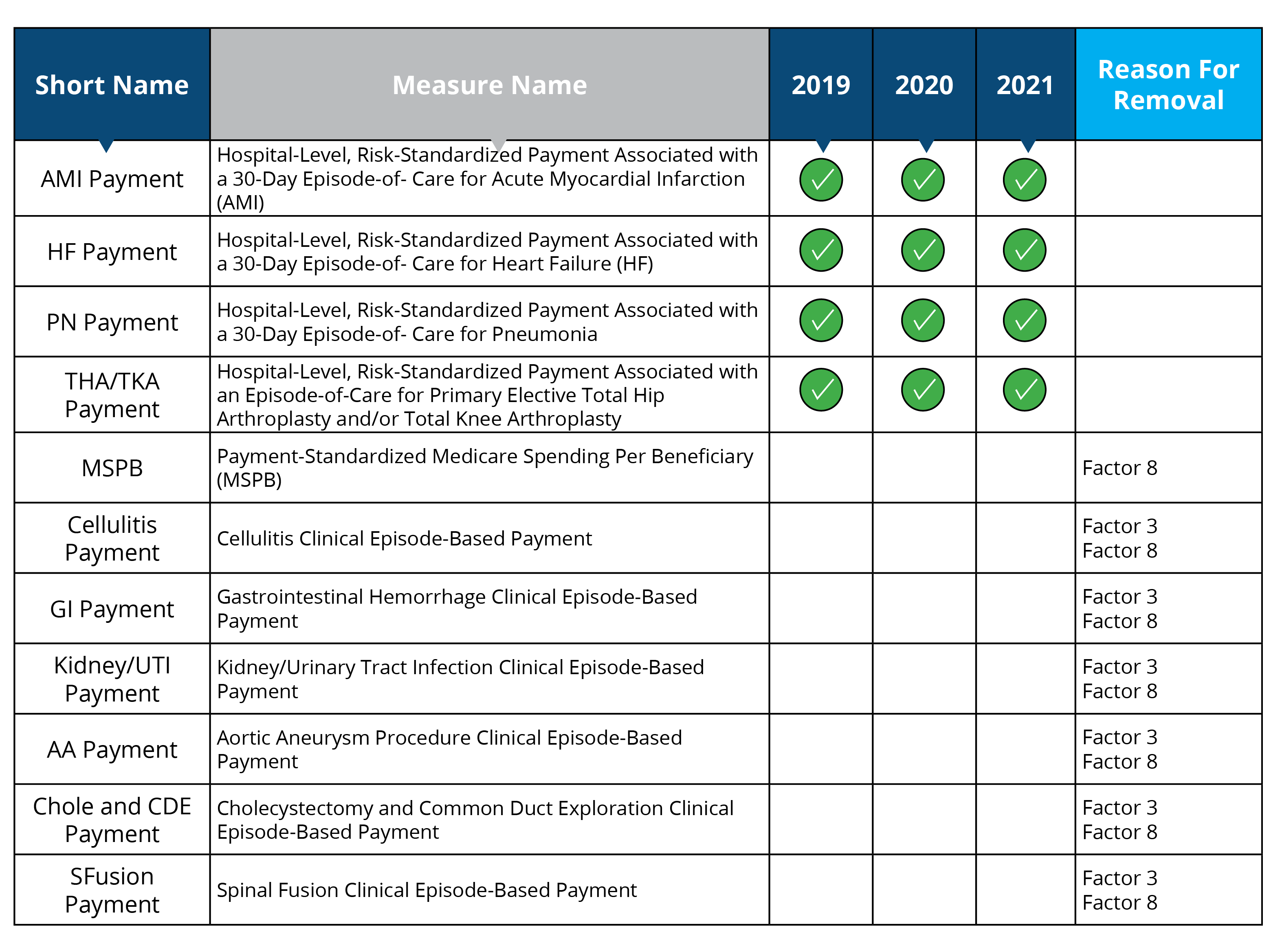
Claims-Based Payment
Measures removed starting in 2019: MSPB, Cellulitis Payment, GI Payment, Kidney/UTI Payment, AA Payment, Chole and CDE Payment, SFusion Payment
Measure removal factor 3 and 8:
- Measure data also captured in a more broadly applicable measure (Cellulitis Payment, GI Payment, Kidney/UTI Payment, AA Payment, Chole and CDE Payment and SFusion Payment)
- The costs associated with the measures outweigh the benefits of their continued use in the program (Cellulitis Payment, GI Payment, Kidney/UTI Payment, AA Payment, Chole and CDE Payment and SFusion Payment)
- The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HVBP (MSPB will remain in the HVBP program)
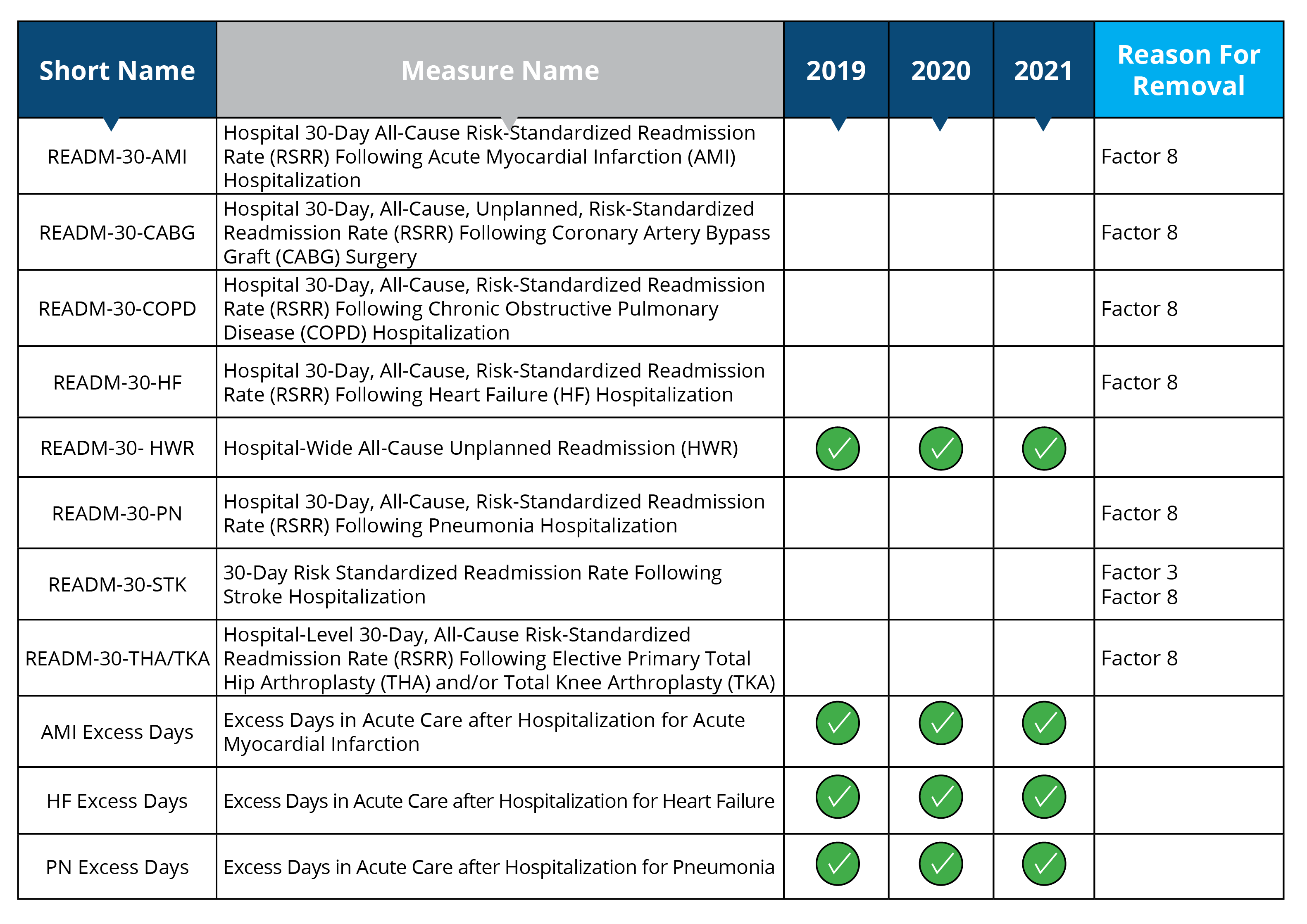
Claims-Based Coordination of Care
Measures removed starting in 2019: READM-30-AMI, READM-30-CABG, READM-30-COPD, READM-30-HF, READM-30-PN READM-30-STK, READM-30-THA/TKA
Measure removal factor 3 and 8:
- Measure data also captured in a more broadly applicable measure (READM-30-STK)
- The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HRRP (READM-30-AMI, READM-30-CABG, READM-30-COPD, READM-30-HF, READM-30-PN READM-30-STK and READM-30-THA/TKA will remain in the HRRP program)
 Minimal Burden Reduction:
Minimal Burden Reduction:
In this category, you can expect to have your reporting burden minimally reduced. While these measures are being removed specifically from the IQR program, they aren’t going away completely. That means you'll still have to report and submit them to a different program.
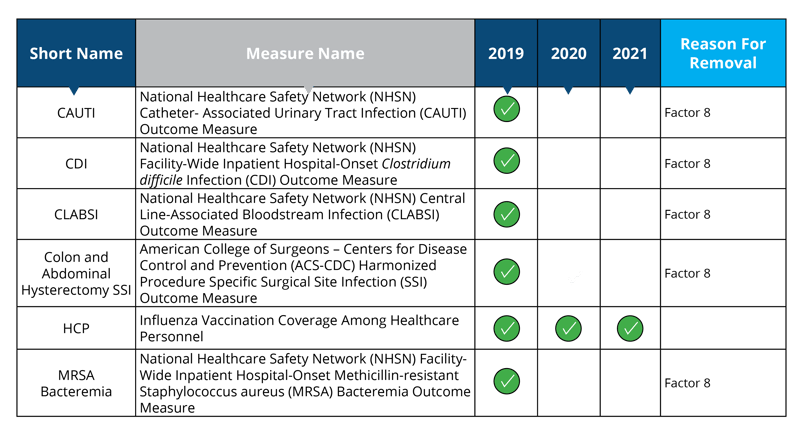
NHSN (Healthcare-Assoicated Infection Measures)
Measures removed starting in 2020: CAUTI, CDI, CLABSI, Colon and Abdominal Hysterectomy SSI and MRSA Bacteremia
Measure removal factor 8:
- The costs associated with this measure outweigh the benefit of its continued use; measure is duplicative of measure in HACRP (CAUTI, CDI, CLABSI, Colon and Abdominal Hysterectomy SSI and MRSA Bacteremia will remain in the HACRP program)
As you can see, the newest measure removal factor addition will have a great impact on the number of measures being removed in 2019 and the reporting burden on your hospital.
Keep in mind that Medisolv doesn’t just provide software. We also have clinical experts who can work closely with you and your team to provide a better understanding of the yearly regulatory reporting changes, including the removal of measures.
Send us a note. We’d love to chat and see how we can help with all of your quality reporting needs.
 |

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments