The Outpatient THA/TKA PRO-PM Measure: A Measurement & Reporting Guide
.png?width=900&height=600&name=BlogImage_THATKAPROPM%20(3).png)
For years, CMS talked about delivering measures to the public that reflected the patient’s assessment of how they fared after interacting with a healthcare organization. Well CMS finally delivered and gave birth to triplets that they named THA/TKA PRO-PM. The first was the Inpatient version and following closely behind were the Outpatient and Ambulatory Surgery Center (ASC) versions. In this article we review the Outpatient THA/TKA PRO-PM and provide you a comparison between the sister measures.
The full name of the outpatient measure is the Risk-Standardized Patient-Reported Outcome-Based Performance Measure (PRO-PM) Following Elective Primary Total Hip Arthroplasty (THA) and/or Total Knee Arthroplasty (TKA) in the HOPD Setting (THA/TKA PRO-PM). It’s the first time that CMS’s Outpatient Quality Reporting (OQR) program will rely directly on patient input to calculate your performance in a specific measure.
On a tactical level, THA/TKA PRO-PM has been specifically designed to measure your hospital outpatient department’s (HOPD’s) rate of improvement in patients’ self-reported pain and function following elective primary THA/TKA. But CMS’s ultimate hope is that this measure will lead to greater “collaboration and shared decision-making between patients and providers.”
While that is all well and good, the THA/TKA PRO-PM measure is...complicated...to say the least. You will likely have to create new pre- and post-op workflows. You will be required to submit a lot of data. And CMS will be using your data—plus some of their own—to risk adjust and calculate the final outcome for you.
The good news: CMS has given you three voluntary reporting periods to get everything into place before mandatory public reporting kicks in in 2028. The urgent news: data collection for the first voluntary period is already underway, so if you haven’t gotten started, now’s the time. And finally, the hopefully helpful news is that we've got a resource for you to download. You'll need to read this article to understand the three tabs it includes.
Download our OP THA/TKA PRO-PM Guide
|
With all of that in mind, here’s everything you need to know to get moving on this trailblazing, slightly hair-raising, measure.
Which Patients are in the Measure?
To be included in your THA/TKA PRO-PM measure data, patients must meet three criteria.
- Enrolled in Medicare fee-for-service
- Aged 65 or older
- Undergoing an elective outpatient primary THA and/or TKA procedure billed as a Part B (outpatient) Medicare claim, including bilateral (same-day) procedures that occur during the same encounter.
CMS will require you to submit complete data sets for, at minimum, 50% of your eligible patients in order to meet your OQR requirements. That means 50% of your patients who had a procedure in your HOPD must complete the pre-op survey and the post-op survey or else you fail OQR and take a penalty.
Which Patients are NOT in the Measure?
The key word in determining if a patient is eligible for the measure is “elective.” If a patient exhibits any of the following criteria, he or she is not considered an “elective” surgical patient, and is therefore ineligible for the measure:
- The procedure is a revision or to address a mechanical complication from a prior THA/TKA procedure
- The procedure involves a concurrent partial hip/knee arthroplasty procedure or resurfacing procedure
- The patient has a femur, hip, or pelvic fracture which has triggered the procedure
- That patient has a disseminated malignant neoplasm or a malignant neoplasm of the pelvis, sacrum, coccyx, lower limbs, or bone/bone marrow
- The patient is undergoing simultaneous removal of an implanted device or prosthesis
In the download above you'll find the Included CPT codes and Excluded ICD & CPT codes.
What Data Do I Need to Collect?
As you can see in the detailed chart below, THA/TKA PRO-PM relies on a combination of patient-reported outcome (PRO) data and risk variable data; administrative claims data; and Medicare enrollment and beneficiary data, collected at different points in the patient’s care.
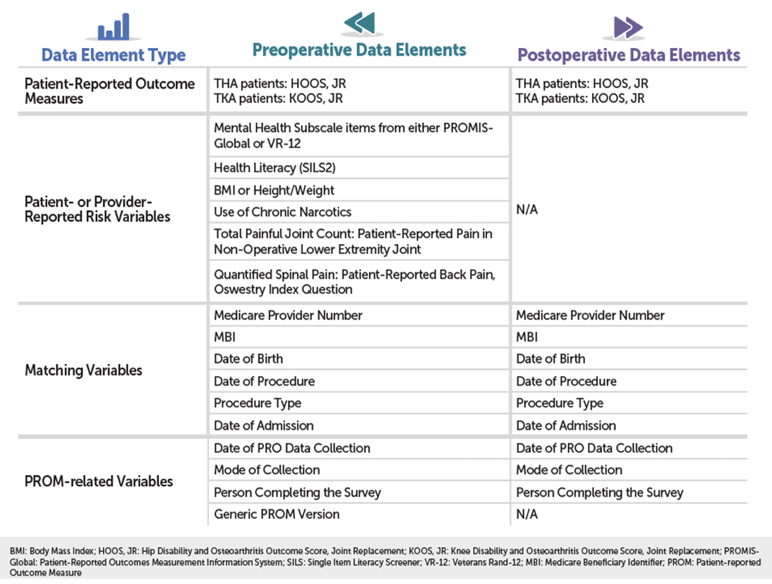
Pre-Op & Post-Op Functional Status Assessments
The heart of your PRO data will be two standardized forms: the HOOS, JR. form for THA patients, and the KOOS, JR. form for your TKA patients. You can download both forms here. Forms will need to be administered to patients two times: once before surgery and once after surgery (see “When Should the Data Be Collected?” for more details). If you’re like a lot of hospitals, these forms are still distributed on good, old-fashioned paper, so may need to manually enter your HOOS and KOOS data after it’s collected.
Pre-Op Risk Variable Data (Patient or Provider Reported)
One other important thing to note: your pre-op data collection process will also need to include a handful of “Patient- or Provider-Reported Risk Variable” screenings that, along with the patient’s 12-month administrative claims history, will be used to risk-adjust your hospital’s outcome.
- Mental Health: PROMIS-Global OR VR-12
-
- How comfortable are you filling out medical forms by yourself?
-
- 0 = Not at All, 1 = A Little Bit, 2 = Somewhat, 3 = Quite a Bit, 4 = Extremely
- BMI OR Height/Weight
- Use of Chronic (≥90 days) Narcotics (See note below)
- Total Painful Joint Count:
-
- What amount of pain have you experienced in the last week in your other knee/hip?
-
- 0 = None, 1 = Mild, 2 = Moderate, 3 = Severe, 4 = Extreme
- Quantified Spinal Pain: Oswestry
-
- At the moment, what is your back pain?
-
- 0 = None, 1 = Very Mild, 2 = Moderate, 3 = Fairly Severe, 4 = Very Severe, 5 = Worst Imaginable
Note on the Use of Chronic Narcotics. We frequently get the question about who is allowed to report the information for this question. Hospitals should rely on collection of this data element by those personnel who they determine can accurately assess and record the information required. These personnel include licensed providers (MD, DO, PA, NP, APRN). Hospitals should follow state regulations to determine which personnel can discontinue medications within their scope of practice, evaluate whether a medication is an opioid, and determine whether the patient is taking the medication for 90 days.
When Should the Data Be Collected?
THA/TKA PRO-PM requires you to collect your PRO data within two very specific windows of time.
|
Pre-Op Data Collection |
Post-Op Data Collection |
|
0 to 90 days before the procedure |
300 to 425 days after the procedure |
IMPORTANT. The Outpatient THA/TKA measure does not have the same reporting cadence as the inpatient version. (How frustrating!)
The OQR measure is based on a calendar year for eligible procedures. And the submission date aligns with the major OQR submission date of May 15 of each year. Remember, this measure has two separate reporting dates which span two separate years.
OP THA/TKA PRO-PM |
||
| Reporting Year (RY) | Encounter Dates | Reporting Deadlines |
|
2025: Voluntary |
Jan 1, 2025 – Dec 31, 2025) |
|
| 2026: Voluntary | Jan 1, 2026 – Dec 31, 2026) |
|
| 2027: Voluntary | Jan 1, 2027 – Dec 31, 2027 |
|
| 2028: Mandatory | Jan 1, 2028 – Dec 31, 2028) |
|
2025 Voluntary Reporting Year: OP THA/TKA PRO-PM |
||||
|
Eligible Procedures |
Pre-Op Collection |
Pre-Op Deadline |
Post-Op |
Post-Op Deadline |
|
January 1 - |
October 3, 2024 – December 31, 2025 |
May 15, 2026 |
October 28, 2025 – March 1, 2027 |
May 15, 2027 |
How Should I Submit My Data?
Medisolv supports this measure in our ENCOR for Hospital Abstracted Measures software. You can learn more about the module and get a quote here. But if you don’t have a vendor like Medisolv, CMS will accept your data as a CSV or XML file, or you can opt to manually enter your data into CMS’s Hospital Quality Reporting system.
How Will My Performance Be Scored?
CMS will compare each patient’s pre-op HOOS/KOOS survey to his or her post-op survey to calculate the change in the patient’s pain and function score. A patient’s change in score must meet or exceed a minimum “substantial clinical benefit threshold” in order to be deemed a success. The threshold is slightly different depending on which survey the patient is taking.
|
Substantial Clinical Benefit Threshold |
|
|
HOOS, JR. |
KOOS, JR. |
|
22 points |
20 points |
Next, CMS will factor in all the risk variable data you submitted, as well as your claims data, to create your final “risk-standardized improvement rate” (RSIR). If, for example, you achieve a 60% RISR, that means that, in general, 60% of your patients report a substantial improvement after their THA/TKA procedure.
Will My Results Be Made Public?
Yes. Your measure scores will be delivered to you confidentially for a preview period first, but results from your mandatory Reporting Year 2028 will be publicly reported—and your annual payment update will be affected.
Where Should I Start?
Chances are, the hardest part of the THA/TKA PRO-PM measure will be determining how and when to collect your pre- and post-op PRO data. Fortunately, CMS has already outlined some helpful workflows for you in a free and easy-to-understand infographic that you can download here.
You can also contact Medisolv for a 1:1 call about our ENCOR software and how we can help with this measure or subscribe to our Education Center for continued updates on as the THA/TKA PRO-PM measure evolves.
Comparing the Measure Across Settings
Finally, I wanted to leave you with a few charts to help you understand the differences between the three sister measures. They are very similar with a few key differences. The first chart includes the encounter and submission dates. The second chart reviews the major differences and similarities between the measures.
| Regulatory Program | Reporting Year | Eligible Encounter Dates | Submission Deadline |
|
IQR |
2025 (Mandatory) |
July 1, 2024 – June 30, 2025 |
Pre-Op Data: September 30, 2025 |
| 2026 (Mandatory) |
July 1, 2025 – June 30, 2026 |
Pre-Op Data: September 30, 2026 |
|
| 2027 (Mandatory) |
July 1, 2026 – June 30, 2027 |
Pre-Op Data: September 30, 2027 |
|
| 2028 (Mandatory) |
July 1, 2027 – June 30, 2028 |
Pre-Op Data: September 30, 2028 |
|
|
OQR & ASCQR |
2025 (Voluntary) |
Jan 1, 2025 – Dec 31, 2025 |
Pre-Op: May 15, 2026 |
| 2026 (Voluntary) |
Jan 1, 2026 – Dec 31, 2026 |
Pre-Op: May 15, 2027 |
|
| 2027 (Voluntary) |
Jan 1, 2027 – Dec 31, 2027 |
Pre-Op: May 15, 2028 |
|
| 2028 (Mandatory) |
Jan 1, 2028 – Dec 31, 2028) |
Pre-Op: May 15, 2029 |
| IQR | OQR | ASCQR | |
| Cohort | Same except procedure billed to Medicare Part A (Inpatient) | Same except procedure billed to Medicare Part B (Outpatient) | Same except procedure billed to Medicare Part B (Outpatient) |
| Submitted Data | Same | Same | Same |
| Reporting Requirements |
Must collect and submit 50% of complete pre- and post-operative data Failure to meet this completion thresholds results in reduction to Annual Payment Update IQR. |
Must collect and submit 50% of complete pre- and post-operative data Failure to meet this completion thresholds results in reduction to Annual Payment Update OQR. |
Must collect and submit 45% of complete pre- and post-operative data Failure to meet this completion thresholds results in reduction to Annual Payment Update ASCQR. |
|
Medisolv Can Help This is a big year for Quality. Medisolv can help you along the way. Along with award-winning software you receive a Clinical Quality Advisor that helps you with all of your technical and clinical needs. We consistently hear from our clients that the biggest differentiator between Medisolv and other vendors is the level of one-on-one support. Especially if you use an EHR vendor right now, you’ll notice a huge difference.
|

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)

-1.png?width=352&name=Blogimage_2026%20OPPS%20Proposed%20Rule%20(1)-1.png)
.png?width=352&name=Implementing%20New%20eCQMs%20A%20Strategic%20Guide%20for%20Hospital%20Quality%20Directors%20(1).png)
Comments