Prepping for Hybrid Measure Submissions

Did you know hybrid measures aren't new? In 2018, Medisolv participated in CMS’s hybrid readmission measure pilot program and as a part of that pilot, we submitted hybrid data electronically for hospitals that we support. There were only 150 submissions nationwide and our clients accounted for 69 of them. Even before that, Medisolv worked with the Yale Center for Outcomes Research and Evaluation (CORE) and CMS to test the feasibility of the hybrid readmission measure. Fast forward to 2022 and we've made it through testing, pilot submissions, a new measure rollout, and we’ve just concluded voluntary submission of the hybrid readmission measure.
Here's what we learned by submitting the hybrid measure data this year and here's some information on each hybrid measure so you can prepare your organization for these new measures and requirements.
Landscape overview
There are currently two hybrid measures that are part of your IQR program requirements. The Hybrid Hospital-Wide Readmission measure (CMS 529) and the Hybrid Hospital-Wide Risk-Standardized Mortality measure (CMS 844).
Both measures are required starting with the 2023 reporting period. The good news is that there is one more voluntary reporting period for your organization to participate in. The voluntary reporting period allows you to review your processes, implement data collection, and “practice” submission before the measures are mandatory and publicly reported. This practice time is critical to improvement and success.
- Voluntary reporting period: July 1, 2022-June 30, 2023 (submission deadline 9/30/23)
- Mandatory reporting period: July 1, 2023-June 30, 2024 (submission deadline 9/30/24)
Submitting the hybrid measure
You may be thinking that it isn’t worth spending time submitting hybrid measure data during the voluntary reporting years, after all, you probably feel pretty comfortable with eCQMs by this point. If hybrid measures were “normal” eCQMs, we might agree with you, but hybrid measures are different than eCQMs and actually aren’t eCQMs at all! So here at Medisolv we are glad we did these submissions for our clients again. We’ve learned a lot over the last few months and expect to learn even more once the Hospital Specific Reports (HSRs) are published next spring.
General differences in submission
- The hybrid measure reporting period and submission window is different than the eCQM cycle. With the addition of the hybrid measures, it is beginning to feel as if we are submitting electronic measures all year long. You will need to plan for these differences in timing, identify resources to support the process and manage the work differently in order to meet the submission requirements.
- You have a shorter window to submit more data. Yup, four quarters of data must be successfully submitted in four separate files in a three-month time span. And that’s if CMS opens the submission window by July 1. This year, that window didn’t open until July 7. Even more reason to track and improve the data that will be included in those files throughout the year. If you wait until after the reporting year ends to validate, you’ll have very limited time to meet the submission requirements.
- The process of submitting files on CMS’s HQR site is a little different. When submitting eCQMs to CMS, hospitals are provided with reports and data that assist in validating the success (or failures) of that submission. You don’t “complete” the submission until all errors, rejections and data mismatches are resolved. The hybrid data submission process on the other hand, provides very little information for validation. The files you submit go into a black hole without much feedback other than “accepted” and “rejected”. CMS currently does NOT provide any additional validation reports or a program certificate. What does that mean to you? You basically have no way to confirm that the data submitted is accurate or complete. You won’t know that until you receive the HSR.
Tips for a successful submission
Just as you need to start working on collecting data for these measures now, you also need to start planning for the submission process now. Even as you are first implementing the measures, well in advance of the submission window, we suggest you analyze the QRDA I files. Confirm that the schema is correct and that there aren’t any obvious issues that will cause rejections and/or errors. Then you should go through that same process again just before the submission window opens. Nope, we aren’t kidding. Just as the data collected and workflow can change throughout the year, so can the QRDA I files.
You'll want to leave adequate time for submission. Complete a final data review and run test submissions before you submit. Here at Medisolv, we do multiple data reviews and test submissions before we even think about submitting to production. Start the submission testing as soon as CMS opens the submission window. Because you must submit eight separate files, four quarters for each measure, the submission process can take a long time, especially if there are errors and rejections that need to be reviewed and corrected. We had some files that needed to be submitted multiple times after errors were addressed.
We’ll let you in on a little secret…below is a list of the most common reasons for rejection and resubmission we saw this year. Keep these in mind and maybe you’ll save yourself some painful resubmissions:
- The end time is before the start time – occasionally the timestamp of a data element defaults to midnight or there’s a typo or it’s not documented at all. When this happens, you can see this error. This is a documentation issue that needs to be corrected.
- There is an incorrect CEHRT ID in the file – one of the most annoying reasons for rejection. If your CEHRT ID is missing or incorrectly documented, your file will reject.
- The QRDA schema is incorrect – review that schema over and over (and over) for accuracy.
- The units of measurement are incorrect – most common problem was “white space” in the unit of measure. Make sure you send a unit of mmHg, not mm Hg (yup, that’s annoying too).
Bottom line? Don’t expect submission to be as easy as an upload and a click of the mouse. Make sure you document the process and report any issues and suggestions to CMS via ONC Jira tickets. We also suggest you establish communication with measure stewards and use other resources.
Remember! If you have Medisolv we do all this for you. Our SubmissionsPlus® Assurance means we take care of all the moving parts of your submission on your behalf.
Other things to consider
- Plan for managing changes to clinical workflow and documentation processes. The Core Clinical Data Elements (CCDEs) and Linking Variables (and those pesky time stamps!) need to be captured properly for all patients. (Information on CCDEs and Linking Variables below.) Many hospitals must change clinical workflows and documentation procedures throughout the year for reasons unrelated to hybrid measure data capture. The IT and Quality teams should work together to communicate those changes, review and update mapping, and provide staff education as needed to improve results throughout the year.
- EHR updates and migrations, server changes and security breaches can all have an impact on your data capture, and ultimately can affect measure results. If you know your organization is planning for an update or migration next year, build in time to validate and troubleshoot technical issues that arise after the fact. Have a plan for how you’ll meet requirements if there is unexpected downtime or a security
- We found that our most successful clients started early, included stakeholders in progress updates and shared successes with their organization.
Was it worth it to submit voluntarily?
Yes. It was worth it.
It was also a pain in the you know what. It was voluntary so it was not a priority for many of our clients, but everyone understood the importance of participating. Hospitals learned about the measure, implemented processes, updated mapping and now have plans for validation and improvement throughout the reporting year. The Medisolv team learned a lot about the submission process, where we can enhance our applications to better support our clients and are better prepared for next year.
Will we do it again? Absolutely - and for both measures next year.
CMS has made it clear they are moving toward a fully digital measurement system. Hybrid measures are technically the first dQM since they are calculated using multiple health information sources.
These measures give us the opportunity to integrate clinical information into hospital quality measurement without manual chart abstraction.
For years, health leaders complained that the claims-only readmissions and mortality measures were not an accurate reflection of how sick their patients were upon entering their facilities. By incorporating clinical data, this provides a fairer picture of the patient's severity of illness.
Now to finish this article, we've put together some measure information to help guide you through hybrid measure set up.
If you are a client and want to participate in voluntary reporting of one or both measures (and you're not already actively participating) reach out to us. If you are looking for assistance with your hybrid and eCQM measure performance, please schedule a 1 on 1 call with us. We'd love to show you how we can help your organization get ready for this digital shift.
What is a Hybrid Measure?
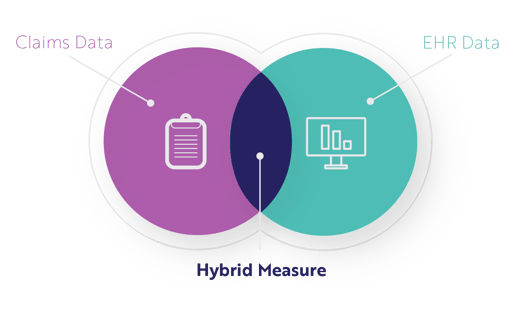
A hybrid measure is a combination of claims data (information submitted for a specific encounter) and EHR data (clinical data). CMS gathers your claims data through the normal process. CMS gets your EHR data through the hybrid measure submissions we just reviewed. CMS then combines the data from each, performs a risk adjustment, and calculates your readmission or mortality performance rate while accounting for the severity of your patient's illness when entering your facility.
The file you submit is a QRDA I file (like your eCQM submissions) but contains two things for each patient.
Core Clinical Data Elements
Core Clinical Data Elements (CCDEs) are patient data points like vital signs (heart rate, weight, oxygen saturation) and lab results (white blood cell count, glucose). These CCDEs reflect the clinical status when the patient first enters the hospital for treatment. They should be routinely and consistently captured in your EHR already, but you may need to update some clinical processes to make sure of that.
CMS needs a way to link those clinical elements with the patient information, so also included in the QRDA I file submission are Linking Variables.
Linking Variables
Linking Variables are the same for each measure. All variables need to align 100% between the patient encounter (claims data), and the CCDEs in the file to count.
Linking variables included in the file submission:
- CMS certification number
- Health Insurance Claim Number or Medicare Beneficiary Identifier
- Date of Birth
- Sex
- Inpatient Admission date
- Inpatient Discharge date
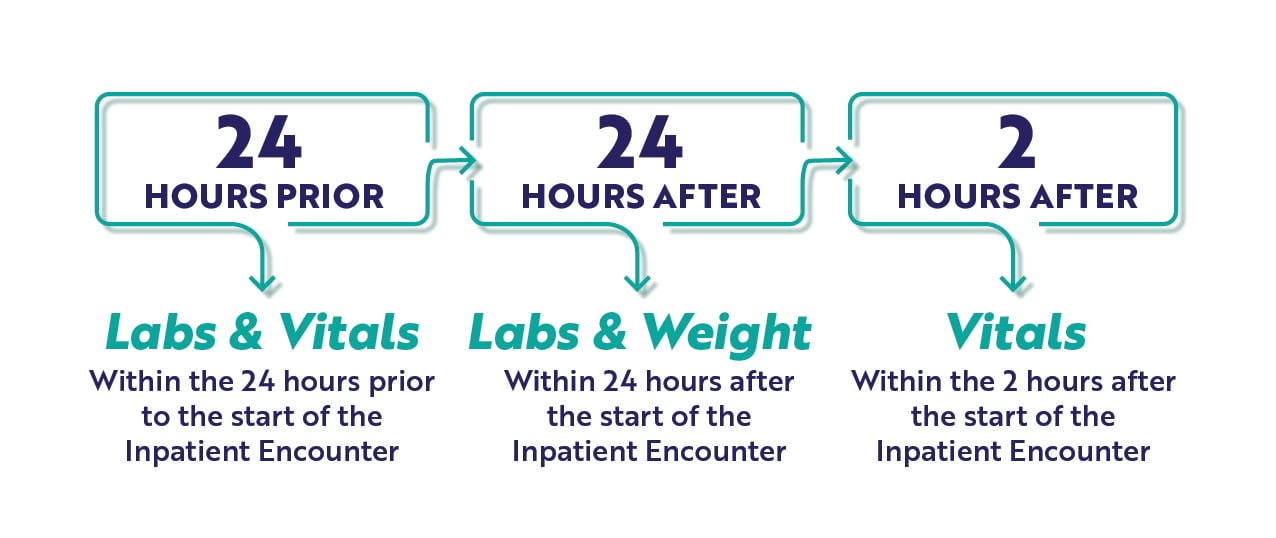
CCDE Timing Requirements
As you review the required CCDEs for each measure, consider the timing graphic below. These measures will capture the earliest instance (<<that's key) of CCDE documentation meeting the timing requirements here. It is very specific and critical to the measure performance which is why we've highlighted this in the graphic below.
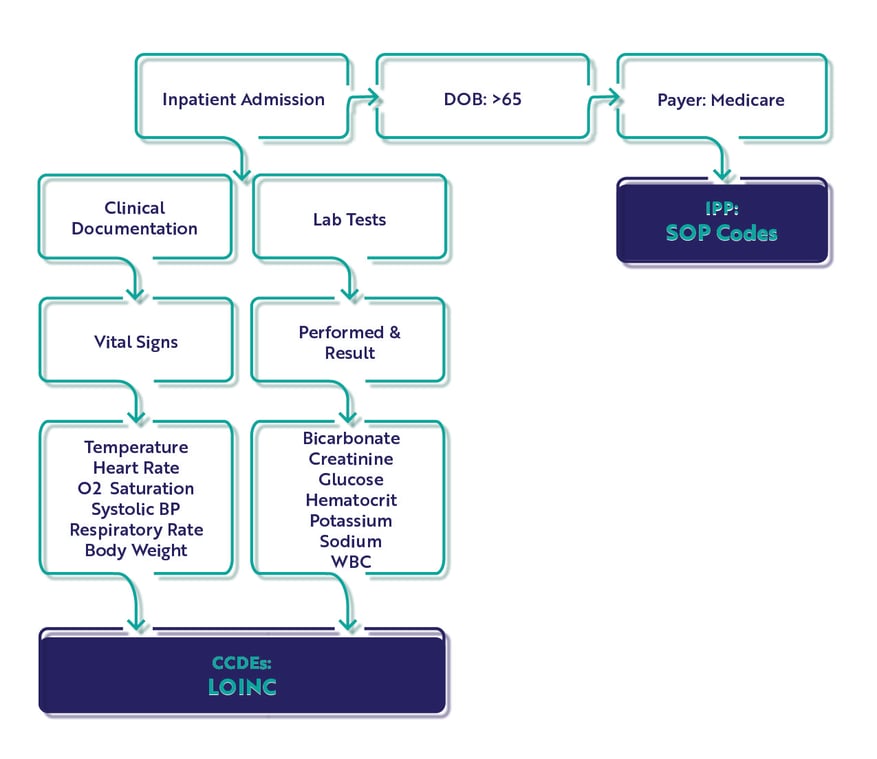
The Hybrid Hospital-Wide Readmission Measure
- CMS 529
- Short Name: HWR
This measure is based on a patient unplanned readmission with 30-days of an inpatient admission and CMS will consider differences in case mix and service mix across all hospitals to come up with a final hybrid risk-standardized readmission rate.
Core Clinical Data Elements
There are 13 CCDEs captured for the hybrid HWR measure.
|
Vital Signs:
|
Lab Test Results:
|
Population
In the measure specification, there is only an Initial Patient Population (IPP) and Denominator. There are no Exclusions, Exceptions or Numerator populations. Remember this is the clinical file which is combined with the claims file. CMS will calculate your score after submission.
IPP/Denominator
- Age >= 65 years
- Acute care hospital inpatient encounter
- Length of stay < 365 days
- Discharge during measurement period
- Medicare patient (primary, secondary)
Workflow
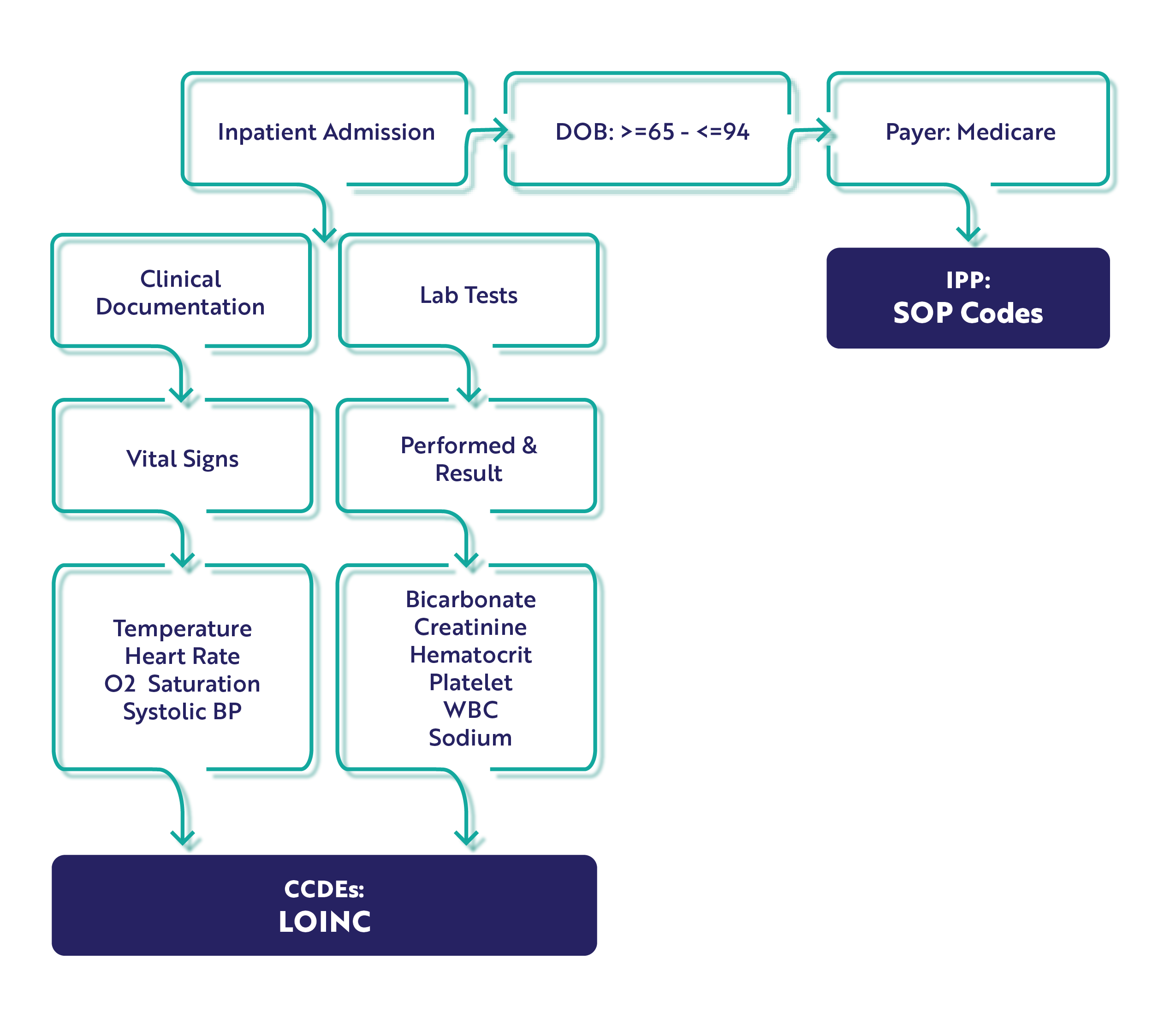
The Hybrid Hospital-Wide Mortality Measure
- CMS 844
- Short Name: HWM
This measure is based on patient mortalities within 30-days of an inpatient encounter and CMS will consider differences in case mix and service mix across all hospitals to come up with a final hybrid risk-standardized mortality rate.
Core Clinical Data Elements
For the HWM measure, there are 10 CCDEs. Please note the addition of platelets to the lab test results CCDE. You'll want to ensure you've got that mapped to the appropriate LOINC code before starting.
|
Vital Signs:
|
Lab Test Results:
|
Population
Just like the hybrid readmission measure, this measure specification has only an IPP and Denominator. Unlike the hybrid readmission measure, the mortality measure has an age range of 65 - 94 years.
IPP/Denominator
- Age >= 65 years to 94 years.
- Acute care hospital inpatient encounter
- Length of stay < 365 days
- Discharge during measurement period
- Medicare patient (primary, secondary)
Workflow
Measure Requirements
To meet the measure requirements, you must include the following, or it will not be accepted.
- Age Requirements: HWR = >65, HWM: 65-94
- Lab Results: must be reported for >90% of non-surgical admissions. If you are a surgical hospital, you need to submit data if available.
- Vital signs must be reported for >90% of hospital admissions
- All six linking variables must be submitted for 95% or more of discharges
If you would like to learn more about the implementation of the hybrid measures, you can watch our on-demand webinar Prepping for Hybrid Measure Submissions. Resident eCQM expert, Kristen Beatson, reviews both measures in depth.
Medisolv Can HelpAlong with award-winning software, each client receives a dedicated Clinical Quality Advisor that helps you with your technical and clinical needs. We consistently hear from our clients that the biggest differentiator between Medisolv and other vendors is the level of one-of-one support. Especially if you use an EHR vendor right now, you’ll notice a huge difference.
|

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments