Five Things to Know About the 2017 IPPS Final Rule

* NOTE Since this blog was written, CMS has updated the requirements for the Inpatient Quality Reporting (IQR) program. To see how the requirements have changed for 2017, please review the changes on this blog: The 6 Things You Need to Know About the 2018 IPPS Final Rule
Changes to the 2017 IQR Program
CMS has issued its Inpatient Prospective Payments Systems (IPPS) final rule for 2017, which makes some significant changes to the Inpatient Quality Reporting (IQR) Program including a mandatory full-year of reporting for eight Electronic Clinical Quality Measures (eCQMs).
Here we’ve highlighted five things to know about the IQR program found in the 2017 IPPS final rule.
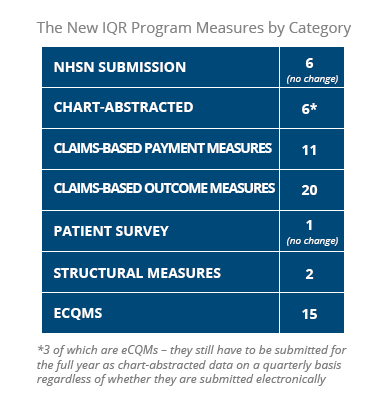
1. Measure changes to the IQR Program
Removing 15 measures
13 are Electronic Clinical Quality Measures (eCQMs) of which two are also chart-abstracted measures. They also removed an additional two structural measures.
Removing 13 eCQMs
CMS has decided to remove 13 eCQMs in 2017 in an “effort to move quality measurement toward outcomes measures.” Many commenters agreed with the removal of these eCQMs saying that the measures were “topped out” and that several of the measures were too complex to capture in electronic form. This should help to decrease administrative burden, minimize confusion among providers regarding data submission and align the IQR Program with other quality measurement efforts.
The final rule made it clear eCQMs are not going away and in fact, they are looking for ways to increase eCQMs over the long term.
“It is one of our goals to expand electronic reporting in the Hospital IQR Program, which we believe will ultimately reduce burden on hospitals as compared with chart-abstracted data reporting and improve patient outcomes by providing more robust data to support quality improvement efforts. We intend to introduce additional eCQMs into the program as eCQMs that support our program goals become available.”
Two of the removed eCQMs are also Chart-Abstracted Measures
In 2017, CMS will remove two measures in their chart-abstracted forms: (1) STK-4: Thrombolytic Therapy (NQF #0437) and (2) VTE-5: VTE Discharge Instructions. They cite the reason for removal is because measure performance among hospitals is so high and unvarying that meaningful distinctions and improvements in performance can no longer be made (‘‘topped-out’’ measures).
Removing two Structural Measures
CMS will remove two structural measures: (1) Participation in a Systematic Clinical Database Registry for Nursing Sensitive Care; and (2) Participation in a Systematic Clinical Database Registry for General Surgery, because performance on these measures does not result in better patient outcomes.
Adding four new claims-based measures
Three are clinical episode-based measures and one is a communication and coordination-of-care measure.
There will be four new measures to the Hospital IQR Program in 2017. CMS stated these “measures … support our mission to provide better healthcare for individuals, better health for populations, and lower costs for healthcare.” These measures are not endorsed by the NQF, but will be submitted for endorsement.
Clinical episode-based payment measures
1. Aortic Aneurysm Procedure Measure
2. Cholecystectomy and Common Duct Exploration Measure
3. Spinal Fusion Measure.
Communication and coordination-of-care measure
1. Excess Days in Acute Care after Hospitalization for Pneumonia
Refinement of two previously adopted measures
- PN Payment: Hospital-Level, Risk-Standardized 30-Day Episode-of-Care Payment Measure for Pneumonia
- PSI 90: Patient Safety and Adverse Events Composite (previously known as the Patient Safety for Selected Indicators Composite Measure)
2. Hospitals must report four quarters of data for eight of the available eCQMs in 2017
CMS has decided to require that hospitals submit eight of the 15 available eCQMs for 2017 and also in 2018. This is a change from the proposed rule which stated that all available eCQMs (15) would need to be reported. CMS decided to adjust their requirements in 2017 after receiving negative feedback about the proposed changes.
CMS said that now is the appropriate time because hospitals have had several years to electronically report data through the Meaningful Use program and the IQR program (three years of voluntary reporting and three years of reporting as part of a pilot). “Based upon data collected by CMS, currently, 95 percent of hospitals attest to successful eCQM reporting under the Medicare and Medicaid EHR Incentive Programs.”
3. Hospitals must complete an annual submission of eCQM data by February 28, 2018
Mostly the same rules of 2016 apply for submission in 2017. Your hospital's experience with 2016 submission should look pretty similar in 2017.
The files your hospital submits for your 2017 data are required to use EHR technology certified to the 2014 or 2015 Edition of CEHRT. In the year after, files must be submitted using the 2015 Edition of CHERT.
Additionally, to align with the Meaningful Use program, the submission deadline for submitting your eight eCQMs to the IQR program in 2017 is February 28, 2018.
4. CMS Expanded The Current Validation Process To Include The Validation Of ECQM Data Beginning In 2018, I.E. 200 Hospitals Will Face An ECQM Audit Of Their 2017 Submission Beginning In The Spring Of 2018
Starting in the spring of 2018, 200 hospitals will be randomly selected for eCQM validation of their 2017 eCQM data, which will determine their 2020 payments. In the past, 600 hospitals were selected for chart-abstracted validation. CMS has added on the eCQM validation in 2018.
What does this mean for you? It means that CMS is thinking seriously about using eCQMs as a determining factor for Hospital Value-Based Programs.
“We believe that as we expand the required reporting of eCQMs in the Hospital IQR Program, we need to validate eCQM data to ensure the accuracy of information submitted by hospitals and reported to the public, as well as for future consideration of eCQMs for potential use in the Hospital VBP Program.”
5. CMS Updated Its Extraordinary Circumstances Extensions/Exemptions (ECE) Policy
CMS will extend the ECE request deadline for non-eCQM circumstances from 30 to 90 days. Additionally, they will establish a separate submission deadline of April 1 following the end of the reporting calendar year for ECEs related to eCQMs.
Other Changes of Note
 Increase of Hospital operating payment rates
Increase of Hospital operating payment rates
Hospitals participating in the IQR and Meaningful Use program will receive a 0.95% increase in their operating payment rate.| (Hospital Market Basket Update) | +2.7% |
|---|---|
| (Multi-factor Productivity) | -0.3% |
| (In accordance with the Affordable Care Act) | -0.75% |
| (Required by the American Taxpayer Relief Act of 2012) | -1.5% |
| (Remove the 2014 Adjustment policy and make up for the effects in 2014, 2015 and 2016. *See sidebar) | +0.8% |
| (Increase to Operating Payment Rate) | TOTAL= 0.95% |
|---|
IQR & Meaningful Use Penalty Assessment
If you do not successfully submit the required quality data to the Hospital IQR Program your hospital will receive a one quarter reduction of the market basket. (Again, the market basket update is 2.7 percent.) Additionally, if your hospital is not a meaningful EHR user, you will be subject to a three quarter reduction of the market basket update.IQR & Meaningful Use Alignment
CMS is working to align the Hospital IQR program and the Meaningful Use program. As a part of this, they reduced the number of eCQMs an Eligible Hospital or a Critical Access Hospital (CAH) must report. They removed 13 eCQMs to align the two programs. Therefore hospitals can submit their eight eCQMs to the Hospital IQR program and get credit for the Meaningful Use program as well.New Notice of Observation
Patients must be notified they are receiving observation services as outpatients for more than 24 hours. Hospitals must explain the “status of the individual as an outpatient, not an inpatient, and the implications of such status.” Both Eligible Hospitals and CAHs are required to furnish a new CMS-developed notice called the Medicare Outpatient Observation Notice (MOON). This will be required to deliver to Medicare beneficiaries who have been receiving observation services as an outpatient for more than 24 hours. The public has the opportunity to comment on the MOON for 30 days after the final rule is published because the notice is going through the Paperwork Reduction Act process.


.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments