eCQM Requirements for The Joint Commission ORYX® Initiative for Quality Improvement

* NOTE Since this blog was written, The Joint Commission has updated their eCQM submission deadline for 2017. The deadline is now June 29, 2018.
We've said it before, and we will say it again; 2017 is an historic time for electronic quality reporting. Have we beat that into your head enough?
For the first time, all major regulatory programs require eCQM submission as a part of successful completion of the program. The following video will provide you with a brief overview of the 2017 eCQM reporting requirements for one specific program, The Joint Commission's ORYX® initiative for quality improvement.
If you don't feel like watching a short video explanation (because you're definitely not a millennial), then feel free to continue reading to get the written recap of The Joint Commission's eCQM reporting requirements.
1. Hospitals must submit four of the available 13 eCQMs
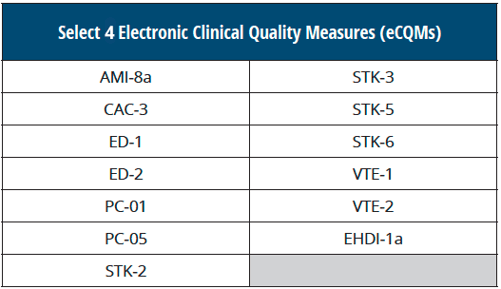
This is the first time that eCQMs are required as a part of The Joint Commission program. Also note, this differs from CMS' requirement for the Inpatient Quality Reporting (IQR) program, which requires four eCQMs from an available list of 15 eCQMs.
Also see: Hospital IQR Program Reporting Requirements for 2017
Additional Options: You may elect to report additional measures relevant to services provided and patient populations served by the hospital.
2. You must submit for one quarter of 2017
The Joint Commission is asking you to dive right into the eCQM world this year. You must report on four eCQMs, and you must report one quarter’s worth of data. We suggest you begin submitting your measures as soon as the submission window opens (October 1, 2017), however, you do have until June 29th of 2018 to get your data submitted to The Joint Commission.
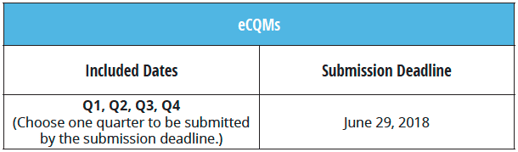
Note: Your data must be submitted no later than 8 p.m. CT on the submission deadline.
3. reporting options
You have two reporting options in 2017. You may either choose to:
a. Use an acceptable ORYX® vendor, like Medisolv, to submit your eCQM data or
b. Complete a direct submission through The Joint Commission portal.
4. Guidelines
The easier option is to use an acceptable ORYX® vendor to submit your eCQMs, but if you choose to direct submit there are a few guidelines you must follow.
a. The data file that you submit must use EHR technology that’s certified to the 2014 or 2015 edition of CEHRT.
b. The EHR technology must be listed on the ONC Certified Health IT Product List.
c. All files submitted to The Joint Commission must be in the QRDA 1 file format.
Keep in mind that if you choose direct submission, then the submission portal will only open for testing in Quarter four of 2017 with the ability to actually submit in early 2018.
Medisolv is a quality reporting solution that helps hospitals and clinicians monitor and submit their eCQMs to various regulatory programs such as The Joint Commission and CMS.
So, if you’re looking for someone to help you with implementation, validation and submission of eCQMs, then send us a note. We’d love to have a chat and see if we are good fit for your needs.
Download:
The 2018 quality reporting bundle
Successfully reporting your eCQM and chart-abstracted measure data to CMS and The Joint Commission is a process that requires some time and preparation. But how can you prepare without the proper information? Do you know what to search for? Is the information that you found correct and accurate for this submission year?
Set your worries aside. We know that you have more important things to do than dig through a handful of confusing CMS and Joint Commission documents—that’s why we did it for you! Yup, true story. We gathered all of the important documents, standardized and simplified them and put everything in our 2018 Quality Reporting Bundle.
Woot woot! *Pause for dance party and sigh of relief.*
Check out what you’ll find in this handy bundle below.
CMS IQR Program:
- Reporting requirements
- Measure list
- Deadlines
The Joint Commission ORYX® Initiative for Quality Improvement:
- Reporting requirements
- Measure list
- Deadlines
Oh, AND an Acronym Reference Guide. No more guessing game.


.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments