5 Hospital IQR Program Requirements in 2017

* NOTE Since this blog was written, CMS has updated the requirements for the Inpatient Quality Reporting (IQR) program. To see how the requirements have changed for 2017, please review the changes on this blog: The 6 Things You Need to Know About the 2018 IPPS Final Rule
If you walk into any mall at this time of the year, you’ll see something very curious. Red and green decorations have crept in among the rows of plaid shirts and brown boots. Fake snowflakes are now dotting windowpanes. Did I just hear Jingle Bells over the speakers? Yes, it appears that the end of 2016 is rapidly approaching.
It may be hard to believe, but for hospitals who have already submitted their Electronic Clinical Quality Measures (eCQMs) to CMS for 2016, it’s time to shift your focus to 2017 electronic quality reporting. This week we review the 2017 hospital IQR program requirements to help your hospital prepare.
The year 2016 was the first time that CMS required hospitals to submit eCQMs to meet part of their Inpatient Quality Reporting (IQR) program requirements. Now in 2017, those requirements have expanded. The changes to the IQR program as outlined in the CMS Final Rule make several adjustments of note to the eCQM requirements.
The Progress of Electronic Quality Reporting
For over 10 years, CMS has been slowly increasing the pressure put on hospitals to come fully into the 21st century with computer technology. This process began in earnest in 2008 with the passing of the Meaningful Use legislation requiring hospitals to document and report the collection of specific data. If hospitals met these criteria, they would be rewarded with stimulus funds.
In 2008, EHR usage was around 10%. By the end of 2014, 76% of hospitals had adopted some form of EHR. Clearly, the program worked.
CMS’ initial focus on eCQMs was only to demonstrate, through Meaningful Use attestation, that the hospital had an EHR in place capable of producing eCQM results. Over the past four to five years, CMS has been subtly shifting to a pay-for-performance model instead. In 2014, CMS introduced the option to submit eCQM results, including patient level detail, electronically via QRDA files. And in 2016, this shift was forcefully demonstrated with the change to mandatory eCQM submission as a requirement for the IQR program.
Article: Review the 2016 eCQM requirements.
Again in 2017, hospitals are required to submit eCQMs. This time, however, the required number of eCQMs doubles and the reporting period has been changed to a full year.
CMS gave no indication that mandatory eCQM submission would slow down either. They stated that now is the appropriate time for mandatory eCQM submission because hospitals have had several years to electronically report data through the Meaningful Use program and the IQR program (three years of voluntary reporting and three years of reporting as part of a pilot).
“Based upon data collected by CMS, currently, 95 percent of hospitals attest to successful eCQM reporting under the Medicare and Medicaid EHR Incentive Programs.” Translation? You’ve said you can do it, now let’s see it in action.
Consequences for Inaction
A word of caution to you. Up to 2% of your Medicare reimbursement funding is at risk. (Check out this infographic showing all at-risk Medicare funding.) If you choose not to submit for 2016, you could lose up to 2% of your Medicare reimbursements in 2018. The same thing applies for 2017. If you choose not to submit 2017 eCQM data, you could lose two percent of your Medicare reimbursement in 2019. Hospitals are looking at a significant decrease in funding over time if they choose not to participate in the electronic submission of quality data.
5 requirements for the CMS IQR program in 2017
Here are the five eCQM requirements for the IQR program for 2017.
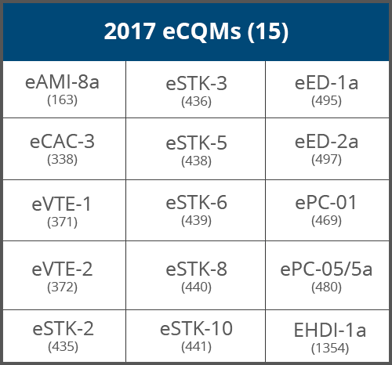
1. Hospitals must choose eight of the available 15 eCQMs to report electronically.
2. Hospitals must report on eCQMs for ALL FOUR quarters of 2017.
3. Submission must be completed by March 16, 2018.
4. The files your hospital submits for your 2017 data are required to use EHR technology certified to the 2014 or 2015 Edition of CEHRT.
5. Data from that period must be submitted in a QRDA I file format.
Keep in mind that this submission for the IQR program is in addition to all of the other existing IQR program requirements hospitals currently fulfill. There is no option, for instance, to reduce the number of chart-abstracted measures that a hospital submits.
What to do now
CMS has decided NOT to publish the 2017 eCQM data on Hospital Compare, but if we look at the history of quality reporting, it will only be a matter of time until results will be posted there. So, it’s prep time.
Quality should review and become familiar with their eCQM data. Get Quality and IT together to start strategizing a plan for next year. Look at the list of available eCQMs above and decide which eCQMs your hospital will focus on. Since The Joint Commission will also require submission of six eCQMs for the ORYX accreditation, we suggest you keep that in mind while choosing your eCQMs.
ON-DEMAND WEBINAR: Tips for selecting and validating your eCQMs.
If you have not already implemented these eCQMs in your hospital, there’s no time to lose. It takes time to properly implement. Not to mention how long it can take to educate and improve compliance with workflow changes.
If you have implemented the eCQMs already, we suggest that you review your results and identify any gaps in performance. Then create a plan for addressing these gaps before or during 2017.
Getting help
We’ve heard a few statements over the course of the year that go something like this, “I’d rather take the penalty then put forward the expense for getting eCQMs up and running.” We hear you. Medisolv has worked with many hospitals from the very beginning of their eCQM process. We’ve felt their frustration and understand their concerns. But we can assure you that we can get you through this process and provide long-term support as the regulations and requirements change.
Not making a plan is still a plan, but not a sustainable one. Yearly penalty assessments will become steeper and accumulate. Now is the time to find a vendor partner that has a long track record of success with eCQMs.
Medisolv’s ENCOR Quality Reporting and Management software solution provides hospitals with the tools they need to meet the CMS reporting requirements.
In addition to the software, our solution provides your hospital with expert clinical consultants that will guide your hospital through implementation, validation and submission. Unlike other companies, we do the heavy lifting for you when it comes to submission.

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments