The eCQM Annual Cycle: Two Processes for your Organization

The start of the year is a time of transition...or is it? Shouldn’t we be able to just roll over into the next year and immediately start tracking what’s happening? Yes, of course. And maybe you're thinking you already do, but the question is, can you track your eCQMs starting on January 1 using the new specifications for 2021?
Each year CMS changes the eCQM specifications which means your vendor has to update your system to the latest specifications for you to see accurate performance rates. It's even more important this year because you must submit two quarters of eCQM data and by 2023 you must submit all four quarters of data.
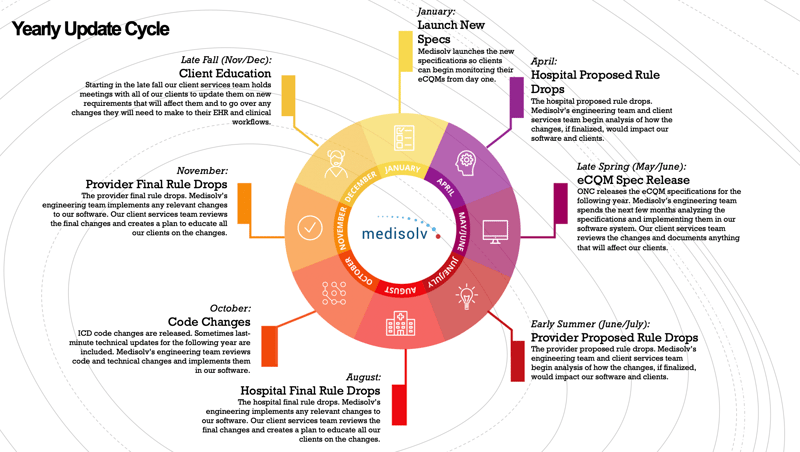
This blog post provides insights into two process we use at Medisolv to track and update eCQMs for hospitals and providers. The first process below is the Medisolv schedule of eCQM specification updates.
Medisolv’s Annual eCQM Specification Update Process
That’s how things work here at Medisolv. Hopefully it’s helpful and provides a list of events to be on the lookout for during the year.
Our second process insight is another annual cycle we’ve built over the years. This one is focused on what a hospital should be doing throughout the year and it goes in four phases.
The eCQM Annual Cycle For a Hospital
Phase 1: Education & Planning
The first step is to understand and define your eCQM goals. Because this process is different for every organization, we can’t exactly attach months of the year to each phase. Generally, Phase 1 is completed in the fall prior to the reporting calendar year - so right now.
If you don’t already have a good team in place dedicated to eCQM monitoring and improvement, put that team together. As a group decide which eCQMs you will focus on improving next year. You can also decide if you will submit the minimum four eCQMs for two quarters or maybe more. Should you begin working on the new opioid eCQM or the hybrid readmission measure next year?
It's best to lay out a clear process for the team to follow. Ensure that you have regularly scheduled meetings to talk about the updates and any ramifications. Make sure everybody understands how those changes are going to impact clinicians and results.
During this time, make sure you review reporting requirements, changes to specifications, updates to the measure logic and/or value sets with your team.
Phase 2: Discovery & Build
The next phase involves undergoing a current state assessment and then building out the necessary changes to facilitate performance improvement. This also takes place usually at the end of the year leading up to the reporting calendar year, but it tends to spill into the next year.
Once you’ve set your goals and reviewed the changes, it's time to assess where you currently stand. What functionalities do you have in place in your EHR? What don't you have in place? Look at your clinical workflows and compare those to where the eCQM is pulling the data. What workflow changes do you need to make in order to capture the data appropriately? What needs to be mapped?
You also want to consider EHR updates. Are you moving to a new EHR? Do you have updates that are coming in? Do you have new functionality that is coming online?
Take a step back and look at all of these items, especially as those new specifications are implemented. Perhaps you've decided to implement the new opioid eCQM next year. If so, you should comb through the measure details and discover how you are currently capturing that data and determine the best way to build what you need to meet that measure. Then move forward by thoughtfully building those updates in your system. Ensure it's a process that works well for both the IT team and the clinicians.
Also see: [WEBINAR] A Review of the New eCQM: Safe Use of Opioids
Review the data. Complete a lot of research. Ask a lot of questions. Revise the processes. Test. Repeat.
This phase is repeated throughout the year. You may find that something is not being documented as it should be and adjustments must be made.
Of course, once you've built the changes you must concurrently train hospital staff. Every update that changes clinical workflow will require additional education.
Phase 3: Evaluation
Once you get through those two major hurdles the process becomes more refined. If your data is mapped properly, it's time to begin evaluating or validating your data.
Make sure you have a good reporting tool (like Medisolv's ENCOR software) that allows you to easily see your data on a regular basis. If a patient failed a measure, drill down to the patient-level details to figure out why they failed. Was it a technical error such as mapping or timing? Was it a compliance issue?
Again, your team will play a critical role in this phase. Ideally, in your regularly scheduled meetings, Quality will present gaps they have noticed in the data. IT will then work on addressing those gaps from the technical side while Quality addresses any compliance issues with the clinicians.
This process begins as soon as you are able and continues for the majority of the year.
Phase 4: Submission
CMS submission usually opens in the fall of the reporting calendar year but the actual deadline is the last day of February of the next calendar year. For most of our hospitals, submission is the easy part. Everything leading up to this phase is where the difficult work takes place.
Here is an example of what a successful eCQM submission to CMS looks like.
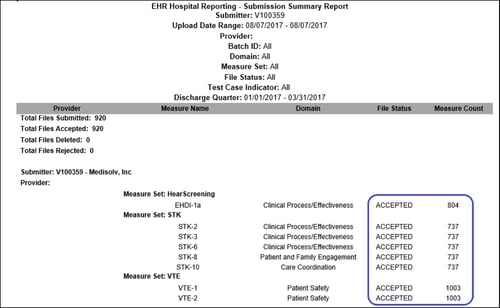
This is what you want to see after you submit to QualityNet. Notice that all files are marked as accepted on the submission summary report.
The process doesn't actually end with submission! We recommend a post-submission validation as well. Using the eCQM Performance Summary Report below, compare what this report says your results were versus what your results were in your eCQM reporting tool. Make sure those results align. If not, work on addressing any gaps in performance.
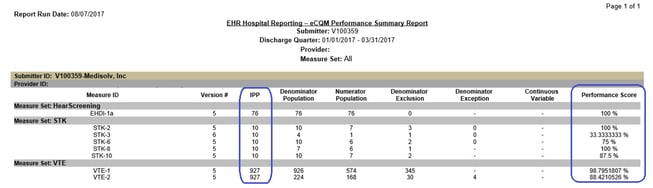
We have a saying here at Medisolv: “Quality is 364 days of the year; submission is one day.” What we mean is that submission should really be a non-event for you, just as it is for our Medisolv clients. You should be focused on using your quality data to make an impactful change at your organization, and you can only do that by having regular access to accurate, meaningful data to measure your performance.
If you would like to talk with us about getting your team on track for successful reporting across all quality programs, send us an email. We'd love to talk to you about how we can help your team.

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments