Medisolv Quality Expert Panel Answers Your FAQs

Do you have questions about Quality reporting? Well you're not alone. In a recent webinar, Medisolv brought together a panel of Quality experts to answer questions from the audience. Participants submitted all types of questions: big, small, specific, broad, procedural, technical, elementary and advanced.
We've pulled some of the best questions from the webinar grouped into four categories below.
Before we begin, let me introduce our panel.

You'll read answers from each of these panel members. Let's begin.
General Quality Questions
Hospital eCQM Questions
Hospital Core Measure Questions
Clinician QPP Questions
General Quality questions
Q: What is the best way to keep educated and up-to-date on all the requirements and the changes for upcoming years?
 |
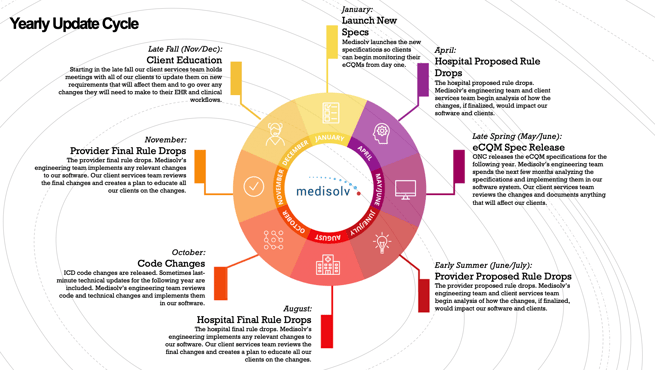
First of all, we here at Medisolv focus on the Quality reporting and the regulatory space and we fully understand how overwhelming it can be. So, to summarize, every year we follow an annual cycle for all the different programs. For example, there is always a proposed rule that is published and then a final rule, and we as vendors get those changes in place as quickly as possible so we can meet the regulatory requirements for our clients. Our goal is to always have the newest and latest specifications available to our clients as early in the year as possible, so that they can be focused on monitoring and improving their performance results and not waiting for new specifications to be released. This is a good schedule for you to keep so you can anticipate when your organization should be preparing, learning and reviewing your requirements. |
Q: Can someone please explain what the hybrid measures are?
 |
There are hundreds of measures available in many different programs, and I’m going on record to say that the hybrid is now my favorite. Medisolv has been working with the hybrid measure since 2014 and was involved in the initial logic reviews and data element feasibility testing. In 2018, CMS opened up voluntary reporting in a hybrid submission pilot. The first thing they realized is that the hybrid measure is not really a measure - there is no rate to be calculated and there is no performance that you are trying to achieve. Think of this measure as a data collection tool. There are 13 core clinical data elements that make up the new Hybrid Hospital-Wide, All Cause Readmission (HWR) measure (6 vital signs, and 7 lab results). The beauty of this measure is that it doesn’t require any additional abstraction and it doesn’t require any additional data documentation. Once you do the initial mapping, all the data elements are collected in your EHR as a part of normal care delivery. The intent of the hybrid measure is to tie clinical data to a claims data for risk adjustment. Think of this measure not as a way to improve performance, but as a way to collect the required clinical data elements to report to CMS, where they can pair the clinical data with an existing claims measure. I would fully anticipate that CMS will do this moving forward to avoid any additional burden for a hospital since there is no additional data collection requirement. So, get ready! If you want to dive deeper into this measure, you can download our free Hybrid Measure Quick Start Guide. |
hospital ecqm questions
Q: Can you please explain the DDS Platform and process? How are your clients handling The Joint Commission's 2019 requirement to use their Direct Data Submission platform for eCQMs? How are you involved?
 |
The Direct Data Submission (DDS) Platform is newly required for eCQM submissions to The Joint Commission this year. Any hospital that is TJC accredited must submit eCQMs through the DDS platform. There is first a registration process, then you must upload the QRDA files to the platform and then troubleshoot any issues. Next, you have to go through a validation process. If issues are found, you have to fix them and then re-upload the data. If you have questions, you should use the Joint Commission’s website as a resource. As for how Medisolv is involved, the process does not change. All of the consultants on our team are fully trained on the DDS platform. They have direct access to your hospital’s platform and will take care of the process of uploading and submitting the data successfully on your hospital’s behalf. Medisolv will continue to support eCQM submissions, while working toward the goal of having good quality data. |
Q: What is the date to attest to eCQMs for Meaningful Use (MU) in 2019?
 |
I’m going to go over a few dates here to make sure we are all up to speed. The Joint Commission: The eCQM submission window through the DDS platform is open now through March 16, 2020. CMS: The eCQM submission window has not opened up yet, but CMS has indicated it will open “sometime this summer” and it will be open through March 2, 2020. The Promoting Interoperability (PI) program (formerly the MU program) submission window opens after the first of the year and runs through March 2, 2020. Just as a reminder, if you are submitting your eCQMs through QualityNet to meet your eCQM requirements for the IQR program, that fulfills your requirements PI program requirements as well. No need to submit to both. |
Q: Do you have a recommended staffing model to support eCQMs? Pre and post implementation.
 |
I wish I had specific numbers to give you, but it really goes back to what’s best for your hospital and the resources you have available. We at Medisolv have some hospitals that have only one or two employees working on eCQMs and others that have 10-20. What you want to consider is the amount of work involved when you are first implementing eCQMs. At Medisolv we provide a lot of hand holding during the initial implementation. In fact, our clients ranked the eCQM implementation phase easier than non-clients in a recent eCQM survey. That being said, there is still a big push in the beginning to get the implementation in place. As time goes on, you’ll find that just having a couple of core teams that meet regularly to go over the annual cycle (above) will work. You’ll need someone from IT and Quality involved, for sure. Initially there will be a handful of people involved, but hopefully as you get your annual process in place, that number will drop off. |
Hospital Core Measure Questions
Q: Where do I find measure specifications for PC and HBIPS?
 |
First, I’ll give you a couple of definitions. PC is the Perinatal Care measure set and HBIPS is the Hospital-Based Inpatient Psychiatric Services measure set. The reason you may have trouble finding these measure sets is because The Joint Commission is the measure steward for the PC and HBIPS measure sets. You’ll find them on The Joint Commission’s website under the measurements tab. You’ll want to download the manual that is specific to The Joint Commission’s measures. |
Q: Are VTE-6, ED-1 and IMM-2 still being collected for CMS and The Joint Commission?
 |
We get this question a lot this year. CMS: The VTE-6, ED-1 and IMM-2 measures were retired beginning with 1/1/2019 discharge. The Joint Commission: The Joint Commission does not require the VTE-6, ED-1 and IMM-2 measures, but they have retained these measures. This means a facility could continue to abstract these measures for internal purposes and performance improvement. You can download our IQR Program Measure Removal Guide for more information. |
Q: What reports should I be running from QualityNet related to my core measure submissions?
 |
One of the most important reports to run through QualityNet is the Provider Participation report. You can run this report for the inpatient measures that are required by CMS. There is also the Potential Duplicates report that will help you identify any duplicates that need resolved before submission ends. Additionally, there is the Population Submission report, the Population and Sampling report, and the Case Status Summary report. Here at Medisolv, we run and provide all of those reports to our clients when we submit their data to CMS. |
clinician qpp questions
Q: What would you suggest for an organization going from one EHR to another mid-year? How can we match demographics and still pass our quality measures?
 |
This truly is one of the biggest challenges on the ambulatory side. If I had to offer quick advice, it would be to try to change EHRs at the beginning of the year, or at the very end of year. CMS does expect you to submit one full-year of data, even if you have two vendors. They expect you to combine and report the data as one submission. I suggest you talk with your two vendors and see if they can provide assistance in combining the data. Although it is a tough and time-consuming situation, Medisolv has been successful in helping clients submit when switching vendors mid-year. |
Q: If you have a variety of provider types – ED providers, PCPs, Nurse Anesthetists – do you recommend picking a few measures for each provider type or picking the best performing measures and letting those scores carry the group?
 |
With each measure you first need to consider if you have an interest in seeing this data on a variety of provider types in order to improve quality. That would be your reason for choosing a variety of providers. If you are trying to maximize your score for MIPS, you will want to change your strategy. You need to figure out which measures you believe you are the strongest in, but you also need to know which measure types you will be submitting (eCQMS, MIPS CQMs or other measures). With this specific example, my advice would be that you pick your best performing measures for the entire group for submission. |
Q: We have new CRNAs joining our TIN in August. How long do they have to be a member of the TIN to be able to group report them?
 |
Luckily, CMS has clarified this. Anyone who joins a TIN between October 1 - December 31 will be included in that TINs group reporting if that TIN currently exists. If they are joining a new practice that is a brand-new TIN, then they would not need to report. Or if they are a new Medicare provider, they would not need to report. |
Is your brain on overload? Are you inspired to ask questions of your own? Good news! Our expert panel is available to answer your questions at any time. If you have a question you would like our expert panel to address, please submit the question to questions@medisolv.com.
|



.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)

Comments