Hospital IQR Program Requirements in 2016
This year your hospital must electronically submit four eCQMs to the Centers for Medicare and Medicaid Services (CMS) Inpatient Quality Reporting (IQR) program. These are 2016 Hospital IQR program reporting requirements:
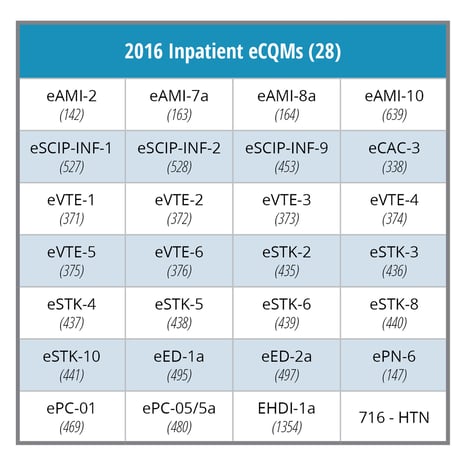
- Your hospital must electronically report on a minimum of four of the 28 available eCQMs.
- Your hospital must report for one quarter (Q3 or Q4) of 2016.
- The submission period for production QRDA files begins in October 2016 and ends in February 2017.
- The data your hospital submits as an eCQM will NOT be posted on the Hospital Compare website.
- The submission of four eCQMs to the IQR program does not replace, chart-abstracted, web-based or claims-based measures, which must still be submitted to the program.
Please note that Critical Access Hospitals are not required to participate in the IQR program. They can continue to fulfill their Meaningful Use program requirements through the Attestation process for 16 eCQMs.
Also note that the IQR program is a penalty program, not an incentive program. If you don’t fulfill these program requirements, your hospital could lose up to two percent of your CMS reimbursements.
If you are unsure of how to select and validate your eCQMs. Check out this blog post with tips for selecting your eCQMs for submission.
Submission services are included with Medisolv's ENCOR Quality Reporting Platform. Contact us today to get started. Email info@medisolv.com.

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments