FAQ: PRO-PM THA/TKA OMG!

Each time CMS releases a new required measure for mandatory submission, there are a slew of questions that follow. But a new measure type takes it to another level. We’ve been fielding questions left and right related to the new mandatory Patient Reported Outcomes-Based Performance Measure (PRO-PM) which is mandatory starting July 1, 2024. We’ve been taking your questions and submitting ticket after ticket to CMS to get you some answers.
In this article, we’ve categorized the questions and tried to edit CMS’s responses to your questions to read in as human a way as possible. We’ve categorized these Q&As as General Measure Information, Survey Collection, Procedure Information, Measure Calculation, Enrollment, Exclusions, and Submission.
We hope you find this resource helpful. If you’ve got more questions, pop them into the comments field.
General Measure Information
- Is the THA/TKA PRO-PM mandatory and if so, when is it required? Will there be an APU penalty if we don’t submit?
- Yes, and yes. Mandatory reporting of the inpatient THA/TKA PRO-PM: Hospital-Level Total Hip Arthroplasty/Total Knee Arthroplasty PRO-PM begins with Fiscal Year (FY) 2027, which translates to July 1, 2024 – June 30, 2025. If you do not meet the measure's requirements, your hospital will get hit with a penalty for your Annual Payment Update (APU).
Also read:
- Is the APU penalty based on BOTH paired PRO collection at 50% of patients or higher AND meeting or exceeding the average score improvement range (i.e., you must meet or exceed BOTH)?
- Only a hospital's response rate impacts the APU. Meeting the measure outcome (whether a patient met or exceeded the Substantial Clinical Benefit (SCB) threshold) is not tied to the APU.
Hospitals must collect and submit complete pre-operative data with matching complete post-operative data for 50% of their eligible THA/TKA inpatients. Hospitals that fail to meet the 50% requirement will receive a reduction in their APU in Fiscal Year (FY) 2028.
- Only a hospital's response rate impacts the APU. Meeting the measure outcome (whether a patient met or exceeded the Substantial Clinical Benefit (SCB) threshold) is not tied to the APU.
- What happens if the hospital does only one inpatient THA/TKA case for the time period and misses out on getting all questions answered for the post-op survey?
- Unfortunately, this would result in a penalty. We asked CMS this question directly a couple times, and each time they responded that it would result in a penalty. “If a hospital performs only one THA/TKA case during the reporting period and fails to collect complete post-operative PRO data for that case, it would not meet the minimum data submission requirement for mandatory reporting.“
- Will there be a low-volume threshold for hospitals who have low volumes of inpatient elective THA/TKA procedures.
- CMS said no. Now let's just see if they revise their answer when the new proposed rule comes out.
- Will there be a zero-denominator declaration for hospitals if they have zero qualifying procedures for the reporting year?
- If a hospital does not perform THA/TKA procedures during the eligible procedure time-period, the hospital would not need to submit any data.
- What is the impact of incomplete surveys on compliance and APU payment?
- Incomplete surveys are not counted towards the 50% requirement for mandatory reporting.
Survey Collection
- What is HOOS JR and KOOS JR?
- HOOS (Hip Disability and Osteoarthritis Outcome Score) and KOOS (Knee Injury and Osteoarthritis Outcome Score) are joint-specific surveys used to evaluate the functional status and pain of THA/TKA patients. These surveys contain questions that assess the patient's hip or knee health on a scale from 0 to 100, where 0 represents total disability and 100 represents perfect health.
- Is there a specified location where you can find the official HOOS Jr./KOOS Jr./PROMIS-Global surveys?
- Yep. Here’s what you need.
- And here are CMS’s questionnaires for the screenings:
- Mental Health: PROMIS-Global OR VR-12
- Health Literacy: Single Item Literary Screener (SILS)-2
-
Quantified Spinal Pain: Oswestry Index Question
- Is data collection for post-op data starts from post-op or from 300–425 days post-surgery?
- Post-operative PRO data is collected 10–14 months (300–425 days) after an eligible THA/TKA procedure.
- Are all data elements required? How should non-responses be handled in the xml? Will there be an issue submitting files with no response?
- Hospitals are encouraged to collect and submit as much data as possible during voluntary reporting of the THA/TKA PRO-PM to become familiar with data submission and the measure.
Here are the required data elements.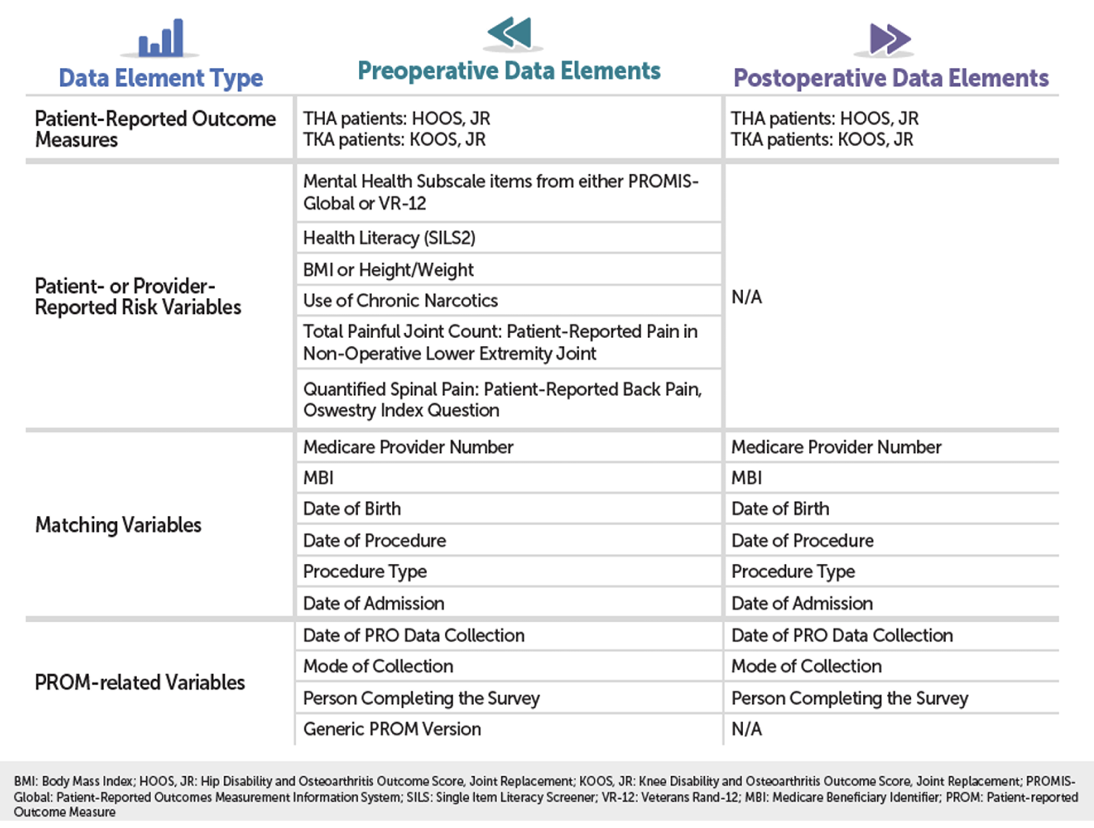
You can also find these in the data dictionary (which lists the data elements, response options, and data collection timeframe) as well as the "What Data Should I Collect" Fact Sheet on QualityNet at: https://qualitynet.cms.gov: Hospitals – Inpatient > Measures > THA/TKA PRO-PM > Resources.
If a patient misses a response to a data element, you can leave the response blank in the XML file. CMS did acknowledge that some patients may choose not to answer some or all questions. XML files will only be rejected if there are issues with:- The root element (IPPRODATA)
- Medicare Provider Number (CCN)
- Medicare Beneficiary Identifier (MBI)
- Survey Type (S_TYPE)
- Procedure Type (P_TYPE).
- Hospitals are encouraged to collect and submit as much data as possible during voluntary reporting of the THA/TKA PRO-PM to become familiar with data submission and the measure.
- On collecting the PROMIS Global data, should we use version 1.1 or version 1.2?
- Either version 1.1 or 1.2 can be used to collect PROMIS Global data for the THA/TKA PRO-PM measure.
- Is the data for only one survey (VR-12 or PROMIS) accepted per patient?
- Hospitals will only need to collect and submit either the mental health-related questions on the VR-12 OR the PROMIS-Global. Hospitals do not need to collect and submit responses for both surveys.
- According to resources documents, VR-12 and PROMIS are only performed pre-op, but the methodology states they are done both pre and post-op. Which is correct?
- Hospitals will only need to collect and submit the mental health-related questions on the VR-12 or the PROMIS-Global pre-operatively. During measure development, these surveys were evaluated at both pre-op and post-op. Since mental health-related questions are used as a risk variable, they only need to be collected pre-operatively.
- Is there a requirement for the "Use of Chronic Narcotics" data element be asked/collected by a clinician (RN or LIP)? If so, are there any recommendations for collection since the other questions are patient-reported?
- The "Use of Chronic Narcotic" data element should come from the medical record/EHR (provider reported).
This data element requires an evaluation by a healthcare provider that the patient either meets or does not meet (yes or no) the definition for the "use of chronic (≥90 day) narcotics. This data element is defined as having any daily or regular intermittent dose of morphine (or a hydromorphone equivalent) for at least 90 days. This definition intends to capture patients with severe pain requiring chronic narcotics prior to THA/TKA procedures.
Hospitals should rely on collection of data elements by those personnel who they determine can accurately assess and record the information required. We leave it to individual surgeons or healthcare providers to determine whether the medication the patient is on is a narcotic and whether very short replacement narcotic use warrants coding as chronic narcotic use for the purposes of collecting this variable. Providers should be collecting data that reflects overall narcotic use (or any narcotic use), not just narcotic use specific to joint pain.
- The "Use of Chronic Narcotic" data element should come from the medical record/EHR (provider reported).
Procedure Information
- Do the PROM survey and HOOS/KOOS survey have to be completed on the same date?
- The PROM survey and HOOS/KOOS survey do not have to be completed on the same date. They can be collected on different days within the appropriate data collection window.
It is important to note that there is a data element date of survey collection (COLLECTION_DT). This data element is intended to be the date the HOOS, JR or KOOS, JR were collected from the patient pre-operatively and post-operatively. If the required PRO data elements were collected on different dates pre-operatively, use the date the HOOS, JR or KOOS, JR were collected for the COLLECTION_DT data element.
- The PROM survey and HOOS/KOOS survey do not have to be completed on the same date. They can be collected on different days within the appropriate data collection window.
- If both a hip and knee procedure occur on the same visit, how are these cases sent in CSV format? We've been told for bilateral, one side is picked and the PROMIS/VR-12 data would be sent only once with either the HOOS, JR or KOOS, JR for one side. How does this work for a hip/knee visit? Are there two lines in the CSV (same VR-12/PROMIS data), then HOOS, JR for Hip and KOOS, JR for knee? How are these counted for the population? As one visit (patient level), or as two separate procedures - one knee/one hip?
- For multiple procedures (hip and knee) during the same visit: although rare, it is possible a patient may have both a hip and knee replacement during the same visit. In this scenario, hospitals should collect a HOOS, JR response for the hip procedure and a KOOS, JR response for the knee procedure. A patient would only need to respond to the other PRO data (e.g., mental health and health literacy) once.
During data submission, hospitals would submit the responses for each joint replacement, HOOS, JR for hip and KOOS, JR for knee on separate lines and you would use the appropriate selection to denote the procedure type:- 1 = Left Hip Replacement
- 2 = Right Hip Replacement
- 3 = Left Knee Replacement
- 4 = Right Knee Replacement
For the other PRO data elements (e.g., mental health and health literacy), your hospital can submit the same responses on each of the separate lines of the CSV for that patient. - Regarding the measure cohort: a patient with both an eligible hip and an eligible knee replacement during the same visit would be considered in the measure cohort once (single visit).
- Regarding the measure's outcome: the measure would evaluate the substantial clinical benefit (SCB) threshold for the HOOS, JR and KOOS, JR. Provided the patient met the SCB threshold for either, they would be considered in the measure's outcome.
- For multiple procedures (hip and knee) during the same visit: although rare, it is possible a patient may have both a hip and knee replacement during the same visit. In this scenario, hospitals should collect a HOOS, JR response for the hip procedure and a KOOS, JR response for the knee procedure. A patient would only need to respond to the other PRO data (e.g., mental health and health literacy) once.
- If a patient has a procedure on June 25, 2024, and a second on August 15, 2024, these are two different measurement periods and should not be treated as a staged procedure, correct? According to the methodology document, "Patients with staged procedures, defined as more than one elective primary THA or TKA performed on the same patient during distinct hospitalizations during the measurement period."
- Here's CMS's response:
"You are correct, the THA/TKA PRO-PM cohort only considers staged procedures which occur during the same measurement period.
For the voluntary reporting period (July 1, 2023 – June 30, 2024) and the mandatory reporting period (July 1, 2024 – June 30, 2025), the measurement period is 12 months. As per the definition of a staged procedure for the THA/TKA PRO-PM, any patient with more than one elective primary THA or TKA performed on the same patient during distinct hospitalizations during the measurement period would be excluded from the measure cohort.
In the scenario you describe, the June 25, 2024 procedure would be considered in the voluntary reporting measurement period and the August 15, 2024 procedure would be considered in the mandatory reporting measurement period. So this patient would be eligible for patient-reported outcome (PRO) data collection and submission (provided they met other cohort eligibility criteria)."
We take this to mean you've got to submit the data for this patient two times during different measurement periods. And that means two PRO data submissions, too. So separate surveys to this patient.
- Here's CMS's response:
- For bilateral procedures performed during the same visit, one survey is filled out, correct?
- In terms of PRO data submission and bilateral procedures, hospitals/entities will not need to submit individual PRO surveys for both the left and right joints (e.g., if there is a bilateral hip procedure on the left and right hip). Instead, you can submit a single set of PRO data and choose to select either the left or right joint for the P_Type response. When CMS combines the submitted PRO data with Medicare claims, CMS will see that it is a bilateral procedure with the submitted PRO data.
- What if two procedures happen on the same visit, but on different days? What is the timeframe for a procedure to be considered staged?
- For multiple procedures on different days (either the same type of procedure, such as both hips or a combination hip and knee): this depends on the timing of the admissions. Specifically, if a patient had a left hip procedure and then the next day had their right hip procedure (same admission), this would be considered a simultaneous bilateral procedure and would be eligible for inclusion in the measure cohort. However, if a patient had a left hip procedure in January and then had their right hip procedure in March (two separate admissions and two separate claims), this would be considered a staged procedure.
Patients with staged procedures are defined as more than one elective primary THA or TKA performed on the same patient during distinct hospitalizations during the measurement period. See performance periods above. - As per the definition of a staged procedure for the THA/TKA PRO-PM, any patient with more than one elective primary THA or TKA performed on the same patient during distinct hospitalizations during the measurement period noted here would be excluded from the measure.
- For multiple procedures on different days (either the same type of procedure, such as both hips or a combination hip and knee): this depends on the timing of the admissions. Specifically, if a patient had a left hip procedure and then the next day had their right hip procedure (same admission), this would be considered a simultaneous bilateral procedure and would be eligible for inclusion in the measure cohort. However, if a patient had a left hip procedure in January and then had their right hip procedure in March (two separate admissions and two separate claims), this would be considered a staged procedure.
Measure Calculation
- Just the HOOS, JR and KOOS, JR questions are counted toward the SCB scoring, correct?
- You are correct, CMS evaluates the patient's response to the HOOS, JR or KOOS, JR to determine if the Substantial Clinical Benefit (SCB) threshold is met.
CMS will compare each patient’s pre-op HOOS/KOOS survey to their post-op survey to calculate the change in the patient’s pain and function score. A patient’s change in score must meet or exceed a minimum SCB threshold to be considered a success. The threshold is slightly different depending on which survey the patient is taking.
Substantial Clinical Benefit Threshold HOOS, JR. KOOS, JR. 22 points 20 points
Next, CMS will factor in all the risk variable data you submitted, as well as your claims data, to create your final “risk-standardized improvement rate” (RSIR). If, for example, you achieve a 60% RISR, that means that, in general, 60% of your patients report a substantial improvement after their THA/TKA procedure.
- You are correct, CMS evaluates the patient's response to the HOOS, JR or KOOS, JR to determine if the Substantial Clinical Benefit (SCB) threshold is met.
Enrollment:
- How do you identify patients? Will you be providing the applicable procedure codes that qualify a patient for the Initial Patient Population?
- We got this question a lot. Short answer – your hospital has to figure out how to identify these patients. CMS has not yet released the applicable procedure codes used to define the measure cohort. They said they will…”in the future.”
In the meantime, if you are familiar with the similar THA/TKA complication measure, the PRO-PM shares a measure cohort with that measure. So, you could take a look at those ICD-10 codes. They are found in Tables 1 and 2 of the 2023 THA/TKA Complications Measure Supplemental File, available on QualityNet at https://qualitynet.cms.gov: Hospitals – Inpatient > Measures > Complication Measures > Methodology.
Then you could go into your scheduling system and identify the patient accounts that have those produce codes and create your list of patients.
You can also review the "Who do I collect PRO data on?" to view a flowchart of how eligible patients are determined for PRO data collection for the hospital-level THA/TKA PRO-PM. This resource is available on Quality Net at: (https://qualitynet.cms.gov) > Hospitals – Inpatient > Measures > THA/TKA PRO-PM > Resources.
- We got this question a lot. Short answer – your hospital has to figure out how to identify these patients. CMS has not yet released the applicable procedure codes used to define the measure cohort. They said they will…”in the future.”
- Does the Medicare patient need to be on Medicare A & B 12 months prior to admission?
- Yes, for inclusion in the THA/TKA PRO-PM measure, patients must be enrolled in Medicare FFS Parts A and B for the 12 months prior to the date of the index admission and enrolled in Part A during the index admission.
- If a patient changes from Medicare FFS to Medicare Advantage, how are they treated? As long as they are FFS during the reference admission and have been for 12 months previously, do they count, even if they switch to Medicare Advantage? Is that correct?
- In the scenario you describe, as long as the patient met the Medicare Fee-for-Service (FFS) enrollment criteria and had a Medicare FFS claim for their THA/TKA procedure, they would be included in the THA/TKA PRO-PM cohort (provided they met all other inclusion/exclusion criteria).
During measure calculation, CMS will use the Medicare enrollment database to evaluate whether patients were enrolled in Medicare FFS Parts A & B for the 12 months prior to the date of admission and Part A during the index admission. An eligible THA/TKA index admission will be included in the cohort if the hospitalization was paid for by Medicare FFS, regardless of whether Medicare is the primary, secondary, or tertiary payor. Inclusion in the measure cohort is driven by the presence of a Medicare FFS claim for an eligible THA/TKA admission.
In terms of identifying Medicare enrollment: hospitals can use their existing billing data to identify a patient's insurance type. If you are unsure of a patient's insurance status, you may submit their data and CMS will determine eligibility.
- In the scenario you describe, as long as the patient met the Medicare Fee-for-Service (FFS) enrollment criteria and had a Medicare FFS claim for their THA/TKA procedure, they would be included in the THA/TKA PRO-PM cohort (provided they met all other inclusion/exclusion criteria).
- What if cases are submitted because they are Medicare FFS, but then it's discovered they were not covered for the previous 12 months prior to admission? Are these surveys thrown out as ineligible?
- CMS gave us sort of a non-answer on this one. Here’s what they said.
“Please note that CMS will evaluate whether patients met the Medicare enrollment requirement (enrolled in Medicare FFS Parts A & B for the 12 months prior to the date of admission and Part A during the index admission) during measure calculation (after hospitals submit PRO data to CMS). If your hospital has already collected PRO data on THA/TKA patients and are uncertain if a patient may be considered eligible or not, we encourage your hospital to submit their data during voluntary reporting and CMS will provide feedback on whether the patient is eligible or ineligible and if ineligible, the criteria for ineligibility.”
- CMS gave us sort of a non-answer on this one. Here’s what they said.
- Is this for Inpatient only or outpatient, too? Should we collect outpatient data now anyway?
- The THA/TKA PRO-PM measure included in the Hospital Inpatient Quality Reporting (IQR) Program is for inpatient settings only.
However, CMS is making this exact same measure a mandatory reporting requirement but for the Outpatient Quality Reporting (OQR) program. That measure is required starting Calendar Year 2028, which begins July 1, 2027.
CMS acknowledged that it may be easier for hospitals to collect PRO data on both inpatient and outpatient procedures given: 1) it may be difficult to identify inpatient and outpatient procedures in advance and 2) it may be advantageous to collect PRO data on the outpatient population because it will soon be mandatory. It’s probably a good idea to start looking at both settings.
- The THA/TKA PRO-PM measure included in the Hospital Inpatient Quality Reporting (IQR) Program is for inpatient settings only.
- Just to clarify--when you say "inpatient" are you referring to the setting in which they had their surgery or their patient status for billing?
- When referring to "inpatient" in the context of the THA/TKA PRO-PM measure, it specifically refers to patients who undergo an inpatient elective primary THA/TKA procedure. The measure does not include outpatient procedures or patients with different patient statuses for billing purposes.
- Can you provide a link to more information about the outpatient version of this measure?
- We really wish we could help here. CMS has not published information about the outpatient version of the THA/TKA PRO-PM measure at this time, except for the 2024 OPPS final rule. It can be found starting on page 440 of the 2024 OPPS final rule, but confusingly it gives you all the wrong dates there because they are talking about what they proposed. In the final rule, they pushed it back one year. Look at page 447 instead, where they say this.
"After considering the comments received, we are finalizing adoption of the THA/TKA PRO–PM into the Hospital OQR Program with modification. In response to interested party feedback, we are delaying implementation of mandatory reporting by one year, such that voluntary reporting would begin with the CY 2025 reporting period and continue through the CY 2027 reporting period, and mandatory reporting would begin with the CY 2028 reporting period for CY 2031 payment determination. The additional year of voluntary reporting would allow time to monitor implementation progress with regards to data collection burden and response rates, as well as time for rulemaking should any improvements for mandatory reporting need to be made."
- We really wish we could help here. CMS has not published information about the outpatient version of the THA/TKA PRO-PM measure at this time, except for the 2024 OPPS final rule. It can be found starting on page 440 of the 2024 OPPS final rule, but confusingly it gives you all the wrong dates there because they are talking about what they proposed. In the final rule, they pushed it back one year. Look at page 447 instead, where they say this.
Exclusions:
- These are just primary joints, correct? Would these include hip conversions?
- The measure includes only elective primary THA/TKA procedures. Patients with fractures and revisions, malignant neoplasms, or mechanical complications are not included. Hip conversions would fall under the category of revisions and would not be included in the measure.
- Is there an opportunity to report patients who declined to participate in the surveys?
- Yes, it is possible to submit patients with incomplete or partial surveys.
- How do we handle patients who are initially booked for outpatient surgery but later change to inpatient status?
- The measure only considers patients who undergo an inpatient elective primary THA/TKA procedure. If a patient is initially booked for outpatient surgery but later changes to inpatient status, their data would be included in the measure.
- If a patient dies during the data collection period, would we still submit that data, or do we exclude the patient?
- The THA/TKA PRO-PM cohort excludes patients who died before completing the post-operative survey. Therefore, if a patient dies within 300 days of the procedure, they would be excluded from the measure cohort. If you are unsure if a patient should be excluded or not, submit their data, and CMS will evaluate the measure cohort inclusion/exclusion criteria during measure calculation after hospitals submit PRO data to determine eligibility.
Submission
- Do you submit both pre-op and post-op data together?
- No, there are separate submission periods for pre- and post-operative data. Pre-operative data is collected within 90 days before the elective primary THA/TKA procedure, while post-operative data is collected 10–14 months (300–425 days) after the procedure.
After each data collection period (pre- and post), hospitals have roughly at least a month to compile and submit their data. The timelines below depict the exact dates for pre- and post-operative collection and submission dates. - Voluntary Reporting:
- Eligible elective procedures: July 1, 2023 - June 30, 2024
- Pre-operative data collection: April 2, 2023 - June 30, 2024
- Pre-Operative Submission Deadline: September 30, 2024
- Post-Operative data collection: April 26, 2024 – August 29, 2025
- Post-Operative Submission Deadline: September 30, 2025
- Mandatory Reporting:
- Eligible elective procedures: July 1, 2024 - June 30, 2025
- Pre-operative data collection: April 2, 2024 - June 30, 2025
- Pre-Operative Submission Deadline: September 30, 2025
- Post-Operative data collection: April 26, 2025 – August 29, 2026
- Post-Operative Submission Deadline: September 30, 2026
The Hospital Quality Reporting (HQR) system is not open for rolling submissions; instead the system will only accept data while the data submission period is open. For example, the Voluntary Reporting 1 post-operative PRO submission period will open in the summer and close on September 30, 2024. Hospitals will receive a communication as soon as the data submission is open. As soon as the data submission is open, hospitals can choose to submit test cases or test files to become familiar with the system.
During data submission, if there are issues with required variables (CCN, MBI, S_TYPE, and P_TYPE), you will receive a rejection message. If there is a missing or invalid value for any of the other variables, you will receive an error message detailing the error, but you will still be able to submit the file. Following voluntary reporting, hospitals will receive more information about their data submission, including their response rates (that may be publicly posted during voluntary reporting). - In terms of data submission, hospitals have the flexibility to submit data through multiple approaches. Hospitals can:
- Send data to CMS for measure calculation directly.
- Utilize an external entity (vendor or registry). Like Medisolv. 😊
- If you go it alone, you must submit the data through the HQR system. The following file formats can be used for data submission: CSV, XML, and manual data entry. Regarding data submission resources: CMS posted a CSV template, CSV file instructions, XML file specifications, as well as a data dictionary, which contains the technical specifications that include each data element and response options.
- For XML Files: you can find the file layout PDF for instructions on XML files
- For CSV Files: you can find the zip file containing a Data Collection Template CSV file and CSV Instructions PDF; you can enter data using the CSV template and can learn more about populating the template in the Instructions PDF
- For Manual Data Entry in the Hospital Quality Reporting (HQR) system: During the data submission period, you can log into the HQR System (https://hqr.cms.gov/hqrng/login), navigate to Data Submissions, and can use the Data Form on the PRO-PM tab.
These resources are located on QualityNet under technical requirements: https://qualitynet.cms.gov/inpatient/measures/THA_TKA/resources.
- No, there are separate submission periods for pre- and post-operative data. Pre-operative data is collected within 90 days before the elective primary THA/TKA procedure, while post-operative data is collected 10–14 months (300–425 days) after the procedure.
Phew! That was a lot of information. We hope this information download was helpful and feel free to post more questions in the comments section below.
Navigating these new PRO-PMs on your own is challenging. Using a vendor like Medisolv to guide you through the regulations and complete the submission on your behalf could prove very beneficial to you and your team. Reach out to us today for a 1:1 call to get the support you need.
Medisolv Can HelpThis is a big year for Quality. Medisolv can help you along the way. Along with award-winning software, you receive a Clinical Quality Advisor that helps you with all of your technical and clinical needs. We consistently hear from our clients that the biggest differentiator between Medisolv and other vendors is the level of one-on-one support. Especially if you use an EHR vendor right now, you’ll notice a huge difference.
|

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments