4 Lessons Learned from Hybrid Measure Submissions

The Hybrid measures are now in their 5th year – yes 5th. The first pilot submission of hybrid data was completed in 2018. Medisolv submitted 69 of the 150 submissions nationwide.
During these five years we have learned a lot about hybrid measures and submissions. This article will review some of the lessons we’ve learned and provide tips for improving your performance for a successful first mandatory year of hybrid tracking and submission.
At the time this article is published hospitals have recently received their 2021 Hospital Specific Reports (HSRs), the 2022 submission window has opened and the 2023 reporting year has begun. Phew! What happened to the lazy, hazy days of summer?
Remember that successfully meeting your hybrid measure requirements is part of your overall IQR requirements. If you don’t meet your hybrid requirements, you don’t successfully complete your IQR requirements which puts 25% of your Annual Payment Update (APU) at risk. 2021 and 2022 are voluntary reporting years. 2023 is required and the performance year starts July 1, 2023, and ends June 30, 2024. Submissions are due by September 30, 2024.
Lesson 1: Linking Variables Matter
Linking the claim to the Core Clinical Data Elements (CCDEs) submitted in the QRDA I file is the first step in the hybrid measure calculation process. CMS will calculate the number of CMS claims files that were linked successfully to a patient’s CCDEs using Linking Variables. If the Linking Variables in the claim file do not match the Linking Variables in the QRDA I file, the patient will “fall out” of the hybrid measure. If any one of the six Linking Variables are missing from a patient’s QRDA I file, the patient will “fall out” of the hybrid measure. Hospitals must successfully submit linking variables for 95% or more of discharges in the QRDA I file. We cannot overstate this: Linking Variables will make or break your hybrid submission.
Areas for Improvement
- Linking Variables are the key to success! Review the linking variable requirements below.
- You must ensure that every Linking Variable is present for every single patient, or it won’t link the claim and the QRDA I CCDE data successfully.
- You must make sure that each Linking Variable is formatted correctly. For eCQMs, when you submit your QRDA I file, CMS provides reports and visibility into errors, rejections, and mismatches. For hybrid measures, it’s quite different. There are very few tools to tell you if you've got it right. You won’t know if there are data issues that impact your ability to meet the requirements, and something as simple as an incorrectly formatted Linking Variable may cause you to fail to meet the requirements. A few examples:
- If you have a different “admission date” in your QRDA I file compared to the admission date in your claim file, you will not get a successful link.
- If you are missing an MBI from a patient record, you will not get a successful link.
- If your QRDA I file is formatted incorrectly or includes incorrect codes, you will not get a successful link.
Lesson 2: Make Sure All Medicare Patient Visits Are Included
We found that in some of the files we submitted for clients, the total Medicare discharges CMS received from the hospital’s claims was different than the number of Medicare discharges submitted in the QRDA I file.
Areas for Improvement
- Make sure you have all payers mapped correctly in your system. If the Source of Payment code for the Medicare Payer is missing, incorrect or inaccurate, the patient will not meet the requirements for hybrid CCDE and will not be included in the QRDA I file.
- Make sure you map and include secondary and tertiary forms of insurance to account for all Medicare beneficiaries.
- Review the patients who qualify for the hybrid CCDE initial patient population. Confirm that the population matches the number of Medicare claims submitted for the year.
- Note: In the 2024 IPPS proposed rule, CMS is proposing to include Medicare Advantage patients too…this is only going to get harder.
Lesson 3: Every CCDE for Every Patient
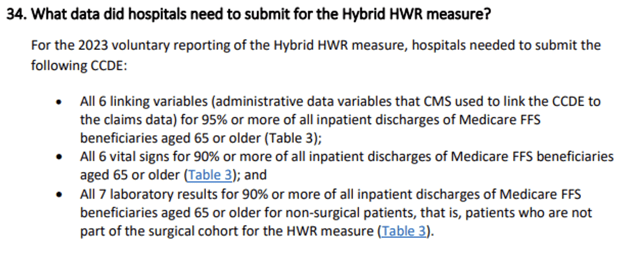
One confusing part of the Hospital Specific Reports that CMS sent back to hospitals was around CCDEs. Our understanding was that hospitals must have 90% of labs and 90% of vital signs included in the file to meet IQR requirements. But if that’s the case, explain this HSR example:
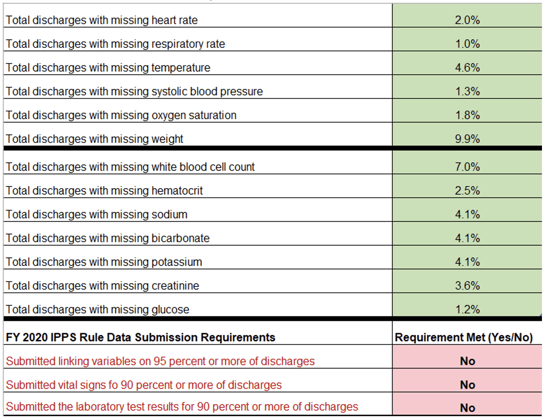
This hospital had less than 10% of each CCDE missing, so how then are they not meeting requirements? Well, the way CMS calculates ‘requirement met’ was not made clear(ish!) until the recent publication of FAQs.
While most of the focus has been on the percent missing at the individual CCDE level (the green in the HSR example above), our interpretation of the new FAQs is that requirement met (red in the example) is based on EACH unique patient encounter having ALL of the labs and ALL of the vital signs documented. Meaning, you could have less than 10% missing from every CCDE but still fail the lab or vital sign threshold requirement because more than 10% of patient discharges were missing at least one lab or one vital sign CCDE.
Areas for Improvement
- Every patient needs to have all required CCDE – all vitals and all labs. You can’t look at just the total missing unique CCDEs and determine if you meet the requirement. You need to look at it as the summary of total CCDEs by patient.
Lesson 4: Units of Measure Matter
Although hybrid resources indicate that any unit of measure can be submitted, findings from the HSR review show that CCDEs were not evaluated and/or converted correctly if the UOM did not align with the standard units:
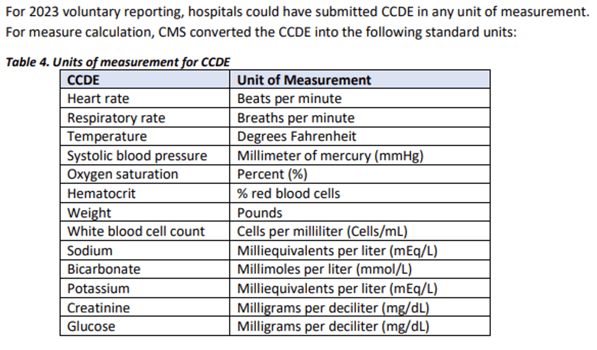
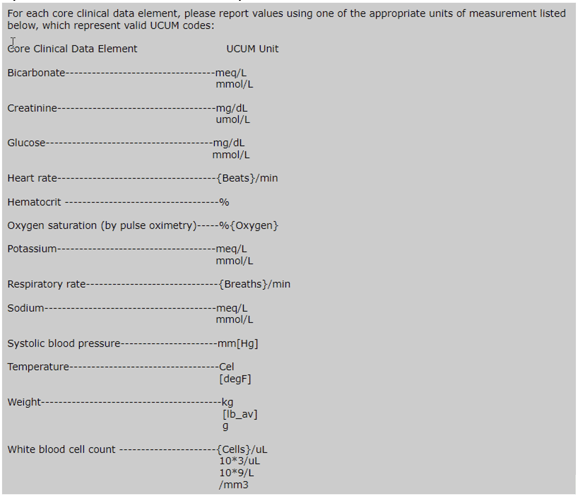
Areas for Improvement
- Every CCDE submitted in the QRDA I file must include a standard unit to ensure complete data evaluation and accurate results. The required units can be found in the measure specifications. Be sure to review the units associated with your CCDE documentation and update to reflect measure requirements.
Hybrid Measure Refresher
We have lots of resources on our blog about how to implement the hybrid measures and some basics if you’re just beginning. Here’s a few links. But I also wanted to point out some of the key requirements for each hybrid measure below.
- How to Implement the Hybrid Readmission Measure
- How to Implement the Hybrid Mortality Measure
- What is a Hybrid Measure?
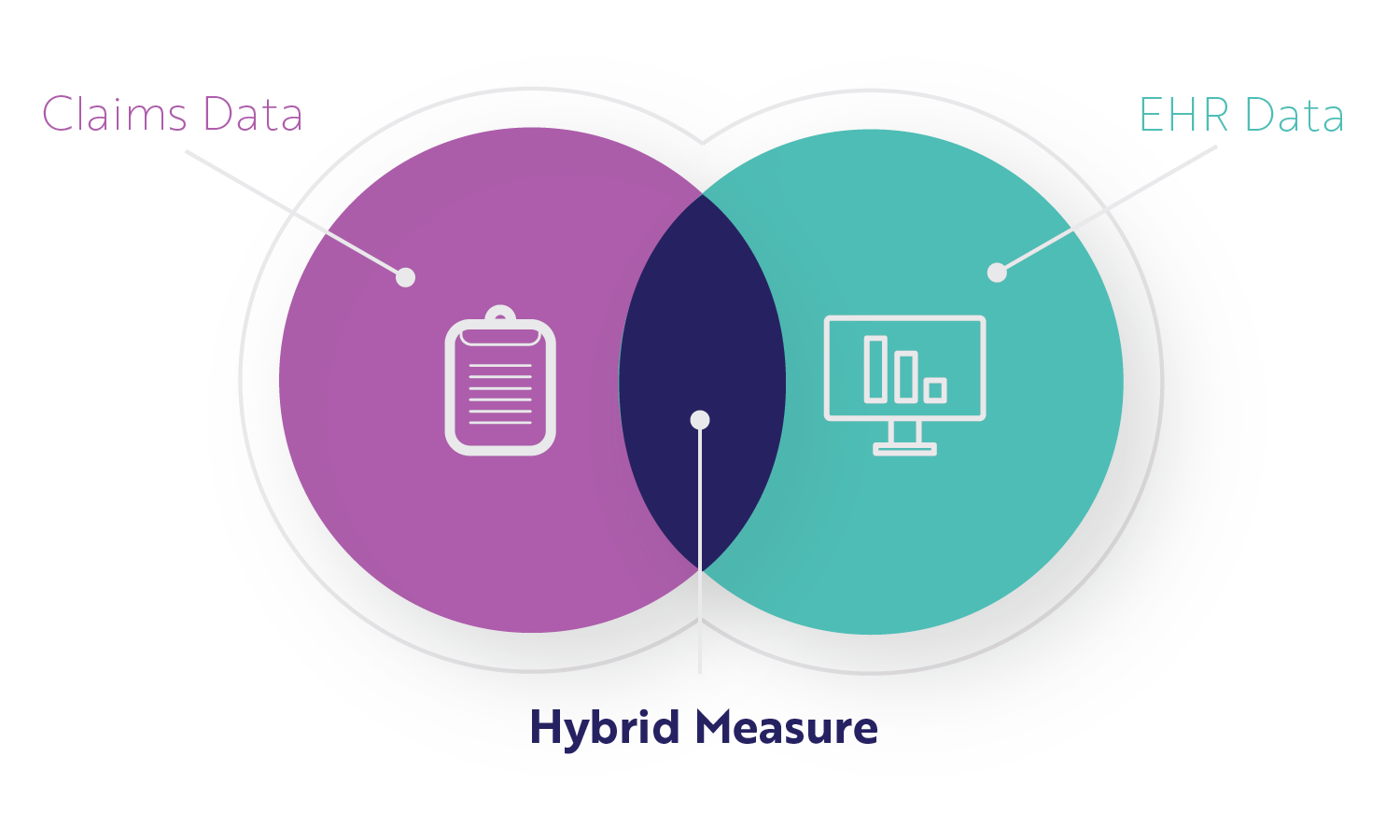
What is a Hybrid Measure?
A hybrid measure is a combination of claims data (information submitted for a specific encounter) and EHR data (clinical data). CMS gathers your claims data through the normal process. CMS gets your EHR data through the hybrid measure submissions we just reviewed. CMS then combines the data from each, performs a risk adjustment, and calculates your readmission or mortality performance rate while accounting for the severity of your patient's illness when entering your facility.
The file you submit is a QRDA I file (like your eCQM submissions) but contains two things for each patient.
Core Clinical Data Elements
Core Clinical Data Elements (CCDEs) are patient data points like vital signs (heart rate, weight, oxygen saturation) and lab results (white blood cell count, glucose). These CCDEs reflect the clinical status when the patient first enters the hospital for treatment. They should be routinely and consistently captured in your EHR already, but you may need to update some clinical processes to make sure of that.
CMS needs a way to link those clinical elements with the patient information, so also included in the QRDA I file submission are Linking Variables.
Linking Variables
Linking Variables are the same for each measure. All variables need to align 100% between the patient encounter (claims data), and the CCDEs in the file to count.
Linking variables included in the file submission:
- CMS certification number
- Health Insurance Claim Number or Medicare Beneficiary Identifier
- Date of Birth
- Sex
- Inpatient Admission date
- Inpatient Discharge date
CCDE Timing Requirements
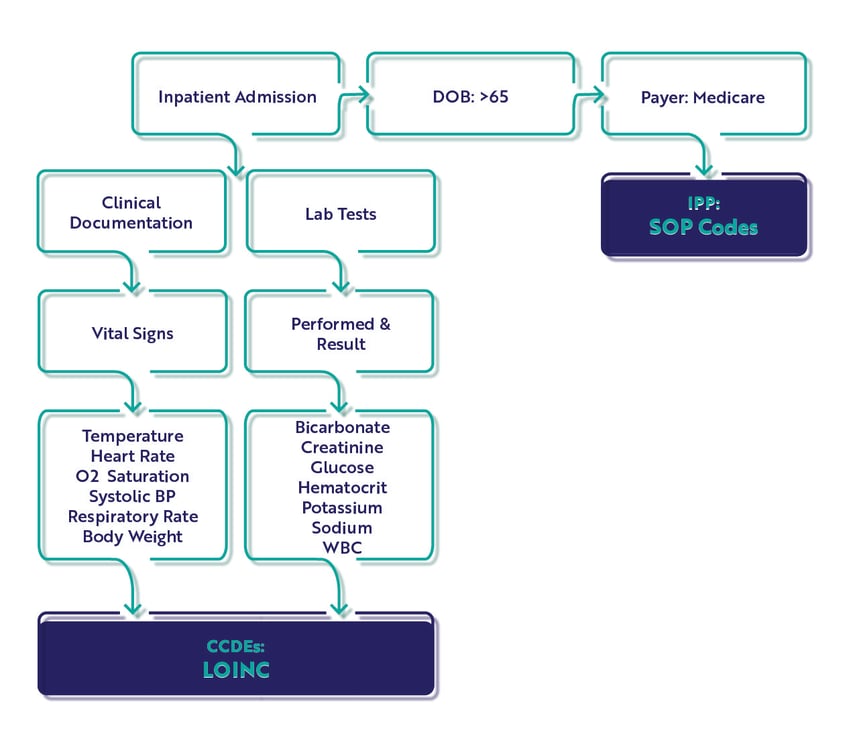
As you review the required CCDEs for each measure, consider the timing graphic below. These measures will capture the earliest instance (<<that's key) of CCDE documentation meeting the timing requirements here. It is very specific and critical to the measure performance which is why we've highlighted this in the graphic below. 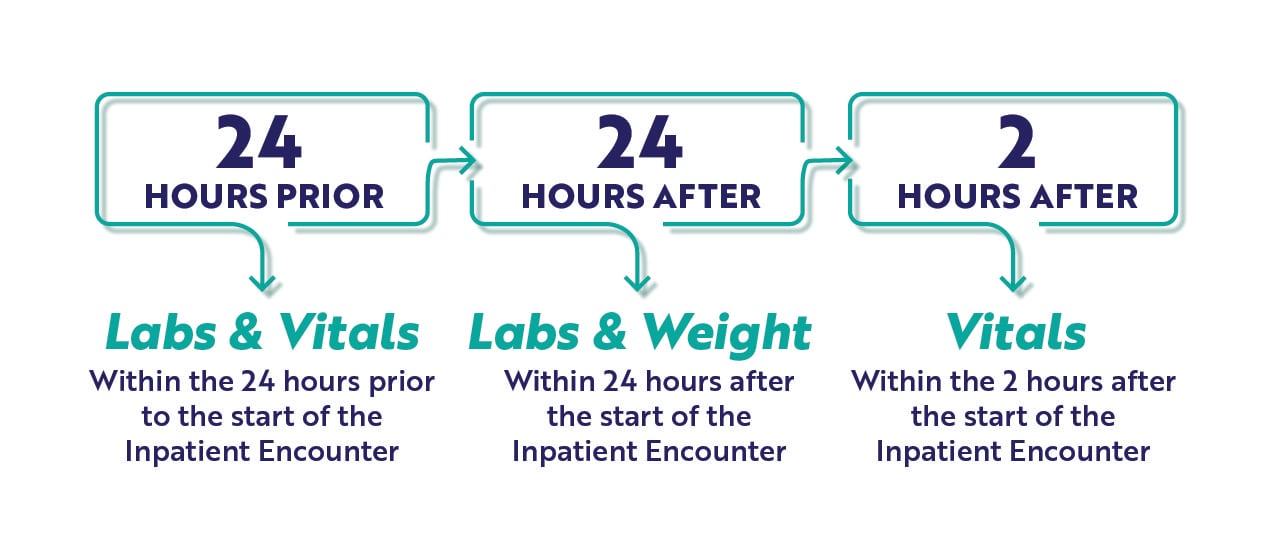
The Hybrid Hospital-Wide Readmission Measure
- CMS 529
- Short Name: HWR
This measure is based on a patient unplanned readmission with 30-days of an inpatient admission and CMS will consider differences in case mix and service mix across all hospitals to come up with a final hybrid risk-standardized readmission rate.
Core Clinical Data Elements
There are 13 CCDEs captured for the hybrid HWR measure.
|
Vital Signs:
|
Lab Test Results:
|
Population
In the measure specification, there is only an Initial Patient Population (IPP) and Denominator. There are no Exclusions, Exceptions or Numerator populations. Remember this is the clinical file which is combined with the claims file. CMS will calculate your score after submission.
IPP/Denominator
- Age >= 65 years
- Acute care hospital inpatient encounter
- Length of stay < 365 days
- Discharge during measurement period
- Medicare patient (primary, secondary)
Workflow
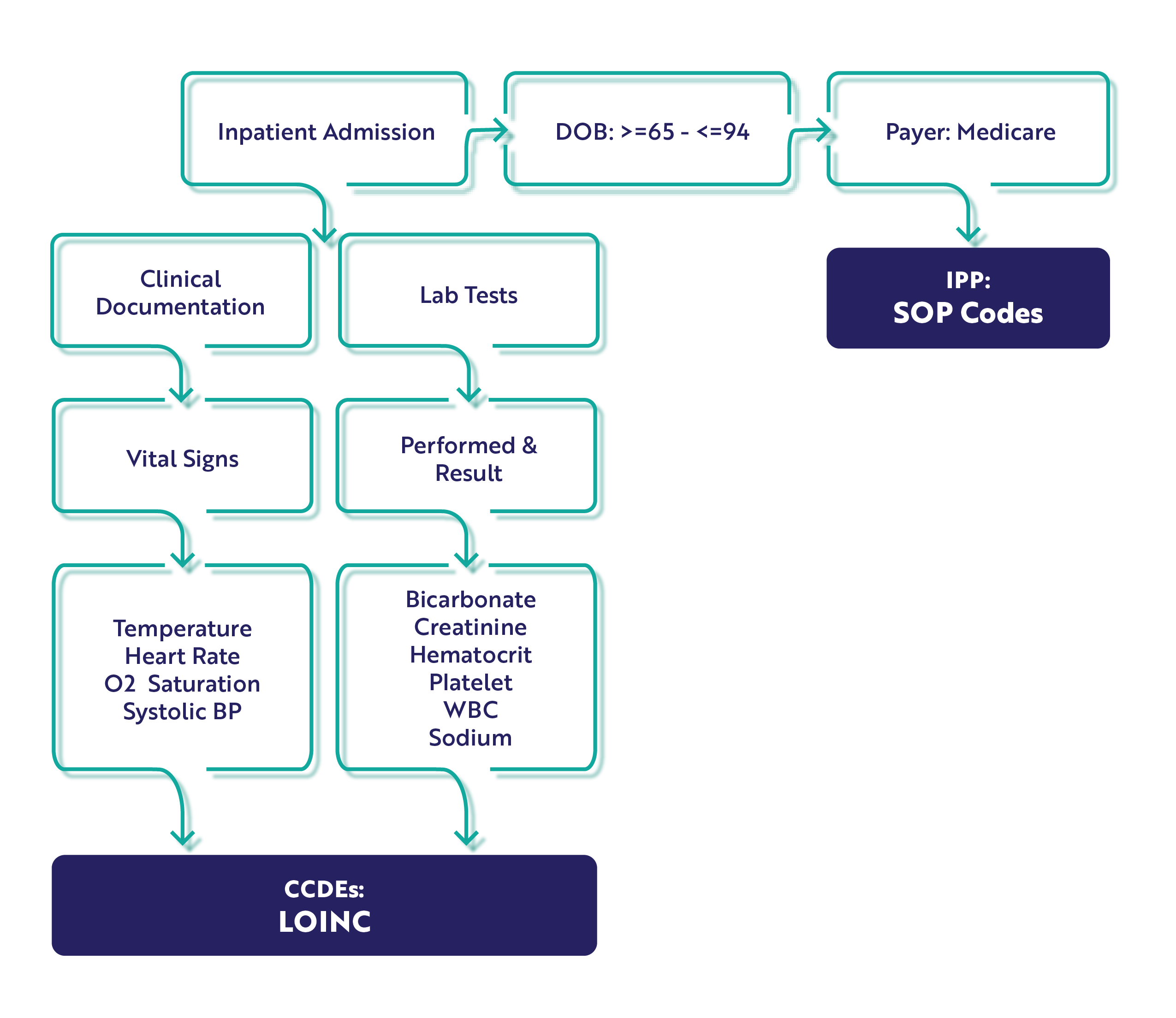
The Hybrid Hospital-Wide Mortality Measure
- CMS 844
- Short Name: HWM
This measure is based on patient mortalities within 30-days of an inpatient encounter and CMS will consider differences in case mix and service mix across all hospitals to come up with a final hybrid risk-standardized mortality rate.
Core Clinical Data Elements
For the HWM measure, there are 10 CCDEs. Please note the addition of platelets to the lab test results CCDE. You'll want to ensure you've got that mapped to the appropriate LOINC code before starting.
|
Vital Signs:
|
Lab Test Results:
|
Population
Just like the hybrid readmission measure, this measure specification has only an IPP and Denominator. Unlike the hybrid readmission measure, the mortality measure has an age range of 65 - 94 years.
IPP/Denominator
- Age >= 65 years to 94 years.
- Acute care hospital inpatient encounter
- Length of stay < 365 days
- Discharge during measurement period
- Medicare patient (primary, secondary)
Workflow
Measure Requirements
To meet the measure requirements, you must include the following, or it will not be accepted.
- Age Requirements: HWR = >65, HWM: 65-94
- Lab Results: must be reported for >90% of non-surgical admissions. If you are a surgical hospital, you need to submit data if available.
- Vital signs must be reported for >90% of hospital admissions
- All six linking variables must be submitted for 95% or more of discharges
If you would like to learn more about the implementation of the hybrid measures, you can watch our on-demand webinar Prepping for Hybrid Measure Submissions. Resident eCQM expert, Kristen Beatson, reviews both measures in depth.
Medisolv Can HelpAlong with award-winning software, each client receives a dedicated Clinical Quality Advisor that helps you with your technical and clinical needs. We consistently hear from our clients that the biggest differentiator between Medisolv and other vendors is the level of one-of-one support. Especially if you use an EHR vendor right now, you’ll notice a huge difference.
|

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments