How to Improve Your Hospital's Contraindication Documentation

This week we are talking about contraindication documentation for eCQMs (or “the dreaded negation,” as we like to call it here at Medisolv). While it’s not exactly a favorite topic by anyone’s stretch of the imagination, having a good process in place for capturing contraindications is one of the key things a hospital can do in order to improve their eCQM results. That’s because most eCQM logic references contraindication which, if documented correctly in the EHR, will prevent the patient from failing the measure and ultimately improve performance.
What is contraindication?
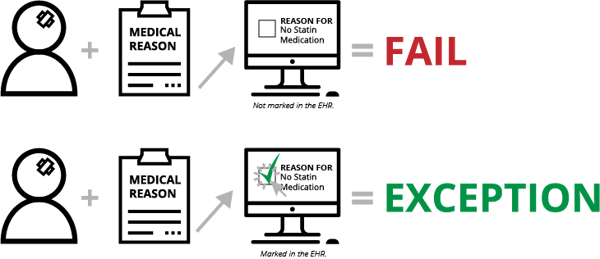
Contraindication is documentation of why something wasn’t done. Depending on the measure, this documentation will put the patient in one of several eCQM populations—Numerator, Exception or Exclusion. For instance, with STK-6 (Discharged on Statin Medication), if the clinician determines there is a medical reason not to prescribe a statin medication upon a patient’s discharge and the clinician doesn't document that reason (in a structured field, mapped to the appropriate codes), the patient will fail the measure. On the other hand, documentation of this reason will qualify the patient for the Exception population and will improve your measure results.
Also see: What Makes up an eCQM?
What makes contraindication difficult?
On its surface, capturing contraindication may not seem complicated. However, this documentation has been difficult to implement and a challenge to sustain compliance.
Why? Well, for clinicians, contraindication falls into the unnecessary burden category. In the past, clinicians might make a note that they didn’t prescribe the statin medication for some reason and an abstractor could use all of the documentation on the patient’s record to assess whether or not there had been some type of contraindication documentation made.
With eCQMs, all documentation must be in a structured field. That means no dictated notes, no free text fields and no sticky notes on a patient’s file. At the very least, it’s one additional click a clinician must make to indicate the reason they didn’t do something (not exactly a time-saver for the clinician).
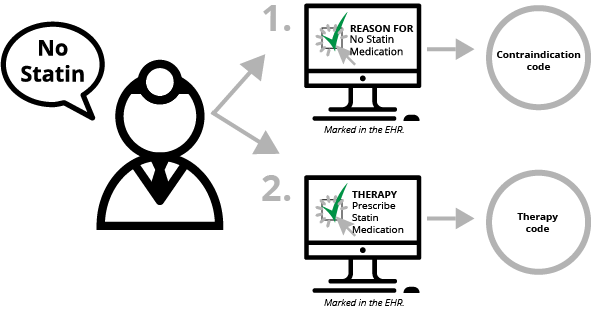
There is one other factor. In addition to documenting why something wasn’t done, contraindication documentation requires clinicians to document what “therapy” (medication order, education, etc.) would have been done if they had not decided to not do the thing they marked they weren’t doing. Yeah, that sentence didn’t make any sense. So here goes.
- Clinician decides not to prescribe statin medication because of a medical reason (contraindication).
- Clinician documents electronically that he or she did not prescribe the medication because there was a medical reason that was contraindicated. This documentation is mapped to a SNOMED code.
- Clinician documents what "therapy" would have been done if the patient had not had a medical reason—i.e the physician clicks "prescribe statin medication." This documentation is mapped to a SNOMED, RxNorm or LOINC code.
You remember OJ Simpson’s terrible book called If I Did It? It’s kind of like that. It makes you think, “Are you kidding me?”
You should have heard me trying to explain this to clients in the early stages of their eCQM implementation journey. I used to start the call with, “Now, don’t hate me…”
The “therapy” documentation is only used for the purposes of submission to CMS for the Inpatient Quality Reporting (IQR) program. It allows CMS to match up the contraindication reason with what therapy is being contraindicated when you submit your eCQM data in QRDA files.
The changing Contraindication process
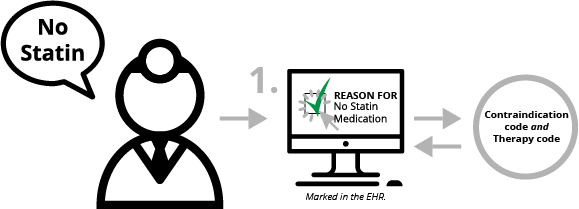
Documenting the therapy that would have been done is one example of documentation that felt unnecessary to the clinician. There is good news though! CMS has changed the requirement for capturing the documentation. Previously, the “therapy” documentation had to be captured with a specific code from the value set, which means a clinician had to select the EXACT therapy they would have ordered. CMS changed this requirement so hospitals can link the “therapy” OID (value set identifier) to the documentation of why a medication was not ordered.
Although a clinician still needs to document the contraindication reason, that contraindication documentation can now link to both the SNOMED code for the contraindication as well as the OID that represents the “therapy” code. So, now the process is much simpler:
- Clinician decides not to prescribe statin medication because of a medical reason (contraindication).
- Clinician documents electronically that they did not prescribe the statin medication because there was a medical reason that was contraindicated. The contraindication documentation is mapped to a SNOMED code and the statin value set OID code – i.e. the “therapy.”
(If you are thoroughly confused, I suggest reading this blog on the elements that make an up an eCQM, taking a nap and then start this blog from the beginning.)
This year, CMS changed all of the OID codes for the "therapy" value sets. This means that if you have not done so already, you’ll need to re-map any contraindication data elements that are mapped to the old (2017) codes so that your eCQM reports can provide accurate results for 2018.
Practices from our successful hospitals
Once you get it all mapped, and you have built any necessary documentation for the physicians, the hardest part about the contradiction process is getting physicians to comply. As you may have read in a previous post, our clients have an 80-90% average rate for their eCQM results. So, this can definitely be done successfully and without overburdening Quality, IT or your clinicians.
Also see: Key Takeaways from 2017 eCQM Reporting
To wrap up today’s post, I’m going to share a few of the best practices from our most successful hospitals.
- Involve the correct people at the onset. If you are just getting started with eCQM implementation or maybe just starting the process of incorporating contraindication documentation, you will want to make sure you include representatives from Quality, IT and most importantly clinicians. When you are making decisions about what a clinician will be required to document, it’s important to have clinician representation to voice their opinion. Between quality and clinician leadership, they can decide where the documentation will make the most sense from a clinical workflow perspective. The IT representatives will be able to decide on the best solution to get it mapped and built technically.
- Schedule regular meetings and educational sessions. As with any type of clinician adoption, it’s best to hold regular meetings and ongoing educational sessions with the people you are requiring to comply with the new documentation request. The lead on the project needs to make sure he or she understands the reasoning behind the contraindication documentation. And the clinicians will need to re-reminded. This requires ongoing education if you want them to comply.
- Prepare in the fall of the previous year. CMS releases new eCQM specification updates fairly early on in the year, which means your team can begin preparing for these changes in the fall of the preceding year. At Medisolv, we interpret the specification changes and then meet with our clients in the last quarter of the year to go over any changes that will affect their eCQMs in the following year. Our best hospitals then gather their teams, review the changes and together with Medisolv, come up with a game plan to make the changes with as few disruptions to clinical workflow as possible.
- Submit to CMS and The Joint Commission as early as you can. Even if you are ready to go on January 1 with the new eCQM changes, you cannot actually implement those changes until you have completed your eCQM submissions to the CMS IQR and The Joint Commission ORYX® initiative for quality improvement programs. For instance, you must use the 2017 eCQM specifications to submit your 2017 eCQM data. At Medisolv, as soon as we complete the submission for our hospitals to both programs, we switch them over to the newest eCQM specification version and begin implementing the plan we reviewed in the fall.
- Monitor your results with frequency. The best way to know if you have a problem with contraindication documentation is by monitoring your results with frequency. If you suddenly have 0% across all of your eCQM results, it’s better to find out you have a problem sooner rather than later. But monitoring isn’t just helpful for technical glitches; by reviewing your numbers consistently, you’ll be able to identify if there are any clinicians who aren’t complying with your documentation requirements.
For more information about how Medisolv can help you with your eCQM needs, check out our ENCOR solution, which is more than just a software. Each hospital is paired up with a Clinical Expert, like me, who will help you navigate tricky elements such as contraindication documentation. Send us an email with any additional questions you have about our solutions.
Get Ahead and Improve Your QualityMedisolv Can Help We've seen some big changes in the CMS eCQMs. With Medisolv as your partner we help guide you through every new change along the way. Talk with us about how we can help you track and improve your quality performance on measures like the safe use of opioids. Blog Post: "What Makes Up an eCQM?" |

.png?width=352&name=2026%20Quality%20Reporting%20Deadlines%20Guide%20(1).png)



Comments